Abstract
Background
Endocrine therapy with aromatase inhibitors (AIs) is the cornerstone of adjuvant systemic treatment for postmenopausal patients with hormone receptor-positive breast cancer. It has become clear that hormone receptor-positive breast cancer carries a consistent risk of relapse up to 15 years after diagnosis. Extended duration of adjuvant AIs therapy after completing initial standard adjuvant AIs-containing therapy may prevent late recurrence and death. We performed a meta-analysis to assess the real impact of the extended adjuvant therapy with AIs.
Methods
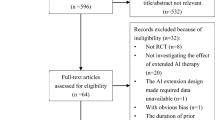
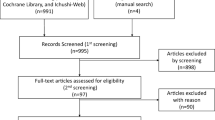
A literature-based meta-analysis of the randomized controlled trials (RCTs) was undertaken. Relevant publications from PubMed, Embase, Cochrane Library, and abstracts from American Society of Clinical Oncology (ASCO) and San Antonio Breast Cancer (SABCS) symposia were searched. The endpoints were disease-free survival (DFS), overall survival (OS), local recurrence, distant recurrence, contralateral breast cancer, non-breast cancer-related death, and toxicity.
Results
Eight trials comprising 15,966 patients met the inclusion criteria. The pooled analysis revealed a significant improvement in DFS (RR = 0.79; 95% CI 0.68–0.91), distant recurrence (RR = 0.75; 95% CI 0.58–0.96), and contralateral breast cancer (RR = 0.53; 95% CI 0.40–0.70) in the extended AIs group. While there was not significant improvement in OS (RR = 1.00, 95% CI 0.99–1.01), non-breast cancer-related death (RR = 1.16, 95% CI 0.96–1.41), and local recurrence (RR = 0.82; 95% CI 0.64–1.06), the subgroup analysis showed that the patient with tumor size > 2 cm (HR = 0.74, RD = − 0.31, P = 0.05 vs. HR = 0.85, RD = − 0.16, P = 0.20), node positive status (HR = 0.77, RD = − 0.27, P = < 0.0001 vs. HR = 0.89, RD = −0.12, P = 0.19) and previous chemotherapy use (HR = 0.75, RD = − 0.29, P = 0.003 vs. HR = 0.91, RD = −0.10, P = 0.44) would get a greater DFS benefit with extended AIs. Longer treatment with AIs was associated with an increased risk ratio of bone pain (RR = 1.26, RD = 0.04, P = 0.003), bone fractures (RR = 1.59, RD = 0.02, P = 0.002), osteoporosis (RR = 1.53, RD = 0.07, P = 0.005), myalgia (RR = 1.26, RD = 0.04, P = 0.02), and treatment discontinuation for adverse events (RR = 1.51, RD = 0.06, P = 0.0009).
Conclusion
After initial standard AIs-containing adjuvant therapy, extended AIs therapy could further bring a DFS benefit for postmenopausal patients with early breast cancer, especially in the patients with high-risk characteristics.





Similar content being viewed by others
Availability of data and materials
All data and materials used in this research are freely available in PubMed, Embase, and Cochrane Library. References have been provided.
Abbreviations
- RCTs:
-
Randomized controlled trials
- ASCO:
-
American Society of Clinical Oncology
- SABCS:
-
San Antonio Breast Cancer
- RR:
-
Risk ratio
- HR:
-
Hazard ratio
- RD:
-
Risk difference
- CI:
-
Confidence intervals
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- N±:
-
Node positive or negative
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- LR:
-
Local recurrence
- DR:
-
Distant recurrence
- CBC:
-
Contralateral breast cancer
- NBCD:
-
Non-breast cancer-related death
- TAM:
-
Tamoxifen
- ANA:
-
Anastozole
- LET:
-
Letrozole
- AIs:
-
Aromatase inhibitors
- OFS:
-
Ovarian function suppression
- AG:
-
Aminoglutethimide
- COMB:
-
Combination
- mo:
-
Month
- plac:
-
Placebo
Reference
Rossi L, Pagani O (2015) The modern landscape of endocrine therapy for premenopausal women with breast cancer. Breast Care 10(5):312–315
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
The Early Breast Cancer Trialists’ Collaborative Group (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient level meta-analysis of randomised trials. Lancet 378:771–784
The Early Breast Cancer Trialists’ Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet 386:1341–1352
Colleoni M, Sun Z, Price KN et al (2016) Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 34(9):927–935
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Mamounas EP, Jeong JH, Wickerham DL et al (2008) Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 26:1965–1971
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816
Gray RG, Rea D, Handley K et al (2013) aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 31(Supplement 1):18
Kaufmann M, Jonat W, Hilfrich J et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol 25:2664–2670
Goldvaser H, AlGorashi I, Ribnikar D et al (2017) Efficacy of extended adjuvant therapy with aromatase inhibitors in early breast cancer among common clinicopathologically defined subgroups: a systematic review and meta-analysis. Cancer Treat Rev 60:53–59
Higgins MJ, Liedke PE, Goss PE (2013) Extended adjuvant endocrine therapy in hormone dependent breast cancer: the paradigm of the NCIC-CTG MA.17/BIG 1-97 trial. Crit Rev Oncol Hematol 86:23–32
Gnant M, Steger G, Greil R et al (2018) A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy—results from 3,484 postmenopausal women in the ABCSG-16 trial. Cancer Res 78(4 Supplement):1
Jakesz R, Greil R, Gnant M et al (2007) Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 99(24):1845–1853
Ohtani S, Lijima K, Higaki K et al (2019) A prospective randomized multi-center open-label phase III trial of extending aromatase-inhibitor adjuvant therapy to 10 years-Results from 1697 postmenopausal women in the N-SAS BC 05 trial: Arimidex extended adjuvant randomized study (AERAS). Cancer Res 79(4 Supplement):1
Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM et al (2017) Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 18(11):1502–1511
Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E et al (2018) Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst 110(1):40–48
Zdenkowski N, Forbes JF, Boyle FM et al (2016) Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptorpositive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial. Ann Oncol 27(5):806–812
Goss PE, Ingle JN, Pritchard KI et al (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375(3):209–219
Mamounas EP, Bandos H, Lembersky BC et al (2019) Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(1):88–99
Burstein HJ, Prestrud AA, Seidenfeld J et al (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28:3784–3796
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 24:2206–2223
Goss PE, Ingle JN, Martino S et al (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262–1271
Cuzick J, Sestak I, Forbes JF et al (1922) Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383(9922):1041–1048
Goss PE, Ingle JN, Ales-Martinez JE et al (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364(25):2381–2391
Howell A, Anderson AS, Clarke RB et al (2014) Risk determination and prevention of breast cancer. Breast Cancer Res 16(5):1–19
Rahman RL, Pruthi S (2012) Chemoprevention of breast cancer: the paradox of evidence versus advocacy inaction. Cancers 4(4):1146–1160
Dubsky P, Brase JC, Jakesz R et al (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 109:2959–2964
Sgroi DC, Sestak I, Cuzick J et al (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14:1067–1076
Gnant M, Filipits M, Greil R et al (2014) Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 25:339–345
Dowsett M, Sestak I, Lopez-Knowles E et al (2013) Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 31:2783–2790
Acknowledgements
We thank Luping chen for her support and guidance throughout the project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
W Ding designed the study and developed the analysis plan. W Ding and X.Y. Qian analyzed the data and performed meta-analysis. Z.A. Li and X.Y. Qian assessed the risk of bias. X.Y. Qian contributed in writing of the article. C.J. Tu and G.D. Ruanuan revised the manuscript and polished the language.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical approval was not applicable for this systematic review and meta-analysis.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, X., Li, Z., Ruan, G. et al. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res Treat 179, 275–285 (2020). https://doi.org/10.1007/s10549-019-05464-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05464-w