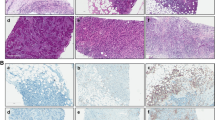
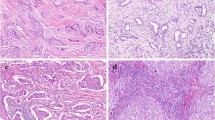
Abstract
Purpose
High-density tumor-infiltrating lymphocytes (TILs) are a prognostic marker for triple-negative breast cancer (TNBC). However, lymphocytic infiltration is heterogeneous in its pattern. We aimed to explore the utility of TIL distribution patterns against TIL density for predicting TNBC prognosis and chemotherapeutic effects.
Methods
Primary invasive TNBC cases were retrieved from a single institutional cohort, and archived samples were reviewed by two board-certificated pathologists. We used 154 consecutive surgical specimens from patients with standard adjuvant therapy, and 80 biopsies taken before primary systemic chemotherapy. The average density of stromal TILs was scored at 10% intervals, while the distribution pattern of TILs was evaluated as diffuse or non-diffuse. The association between TILs and prognosis or pathological complete response (pCR) was statistically analyzed.
Results
A diffuse pattern of TILs at primary surgery correlated with better prognosis (relapse-free survival [RFS], hazard ratio [HR] 3.71, 95% confidence interval [CI] 1.60–8.57; overall survival [OS], HR 3.87, 95% CI 1.46–10.27), as well as high TIL density (≥ 50%; RFS, HR 4.51, 95% CI 2.06–9.90; OS, HR 3.28, 95% CI 1.32–8.14). Diffuse TIL pattern and nodal status were independent prognostic factors in multivariate analysis. Diffuse TIL pattern upon biopsy was associated with higher pCR rate (diffuse, 46%; non-diffuse, 21%; P = 0.032). All high TIL cases had diffuse patterns and the best outcome. Interobserver concordance was moderate (k = 0.53–0.55; distribution pattern) to good (weighted k = 0.67–0.69; density), and it was faster to assess the distribution pattern than to assess the density of TIL.
Conclusions
Showing similar clinical impacts to the TIL density, diffuse TILs could be a predictive marker for better prognosis and higher pCR. The assessment of TIL distribution pattern is simple, faster, and practical. Heterogeneous tumor immunity may contribute to further stratification of TNBC treatment.




Similar content being viewed by others
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- TIL:
-
Tumor-infiltrating lymphocyte
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
- HER2:
-
Human epithelial growth factor receptor 2
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- HE:
-
Hematoxylin–eosin
- PSC:
-
Primary systemic chemotherapy
- LPBC:
-
Lymphocytic-predominant breast cancer
References
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31(7):860–867. https://doi.org/10.1200/JCO.2011.41.0902
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, Andre F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kummel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33(9):983–991. https://doi.org/10.1200/JCO.2014.58.1967
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966. https://doi.org/10.1200/JCO.2013.55.0491
Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, Colleoni AM, Goldhirsch A, Viale G (2016) Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 27(2):249–256. https://doi.org/10.1093/annonc/mdv571
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria S, Gray R, Munzone E, Lemonnier J, Sotiriou C, Piccart MJ, Kellokumpu-Lehtinen PL, Vingiani A, Gray K, Andre F, Denkert C, Salgado R, Michiels S (2019) Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 37(7):559–569. https://doi.org/10.1200/jco.18.01010
Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24(36):5652–5657. https://doi.org/10.1200/jco.2006.06.5664
Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP (2007) Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s surveillance, epidemiology, and end results database. Cancer 110(4):876–884. https://doi.org/10.1002/cncr.22836
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377(6):523–533. https://doi.org/10.1056/NEJMoa1706450
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767. https://doi.org/10.1172/JCI45014
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. https://doi.org/10.1126/science.1203486
Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA (2016) PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 47(1):52–63. https://doi.org/10.1016/j.humpath.2015.09.003
Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, Gartner J, Jia L, Trebska-McGowan K, Somerville RP, Robbins PF, Rosenberg SA, Goff SL, Feldman SA (2018) Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 24(6):724–730. https://doi.org/10.1038/s41591-018-0040-8
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/NEJMoa1809615
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A (2015) Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 26(8):1698–1704. https://doi.org/10.1093/annonc/mdv239
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kummel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50. https://doi.org/10.1016/s1470-2045(17)30904-x
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, VandenEynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TWG (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S (2017) Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol 7:156. https://doi.org/10.3389/fonc.2017.00156
Hida AI, Ohi Y (2015) Evaluation of tumor-infiltrating lymphocytes in breast cancer; proposal of a simpler method. Ann Oncol 26(11):2351. https://doi.org/10.1093/annonc/mdv363
Greer LT, Rosman M, Mylander WC, Hooke J, Kovatich A, Sawyer K, Buras RR, Shriver CD, Tafra L (2013) Does breast tumor heterogeneity necessitate further immunohistochemical staining on surgical specimens? J Am Coll Surg 216(2):239–251. https://doi.org/10.1016/j.jamcollsurg.2012.09.007
Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, Li Z (2017) HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat 166(2):447–457. https://doi.org/10.1007/s10549-017-4453-8
Luen SJ, Savas P, Fox SB, Salgado R, Loi S (2017) Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 49(2):141–155. https://doi.org/10.1016/j.pathol.2016.10.010
Nawaz S, Heindl A, Koelble K, Yuan Y (2015) Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod Pathol 28(6):766–777. https://doi.org/10.1038/modpathol.2015.37
Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y (2018) Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER + breast cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx137
Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, Rai Y, Oshiro Y, Aogi K, Sagara Y, Ohi Y (2016) Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 158(1):1–9. https://doi.org/10.1007/s10549-016-3848-2
Watanabe T, Hida AI, Inoue N, Imamura M, Fujimoto Y, Akazawa K, Hirota S, Miyoshi Y (2018) Abundant tumor infiltrating lymphocytes after primary systemic chemotherapy predicts poor prognosis in estrogen receptor-positive/HER2-negative breast cancers. Breast Cancer Res Treat 168(1):135–145. https://doi.org/10.1007/s10549-017-4575-z
Hida AI, Mizuno Y, Ueda N, Oshiro Y, Sugita A, Matsukage S, Maeda T, Kito K, Ohtsuki Y, Aogi K (2017) Simple assessment reveals the importance of distribution and density of tumor-infiltrating lymphocytes in triple-negative breast cancer. Breast 32:S33–S34. https://doi.org/10.1016/S0960-9776(17)30134-0
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical O, College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. https://doi.org/10.1200/jco.2006.09.2775
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical O, College of American P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/jco.2013.50.9984
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCIEWGoCD (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23(36):9067–9072. https://doi.org/10.1200/jco.2004.01.0454
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F (2014) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 25(3):611–618. https://doi.org/10.1093/annonc/mdt556
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113. https://doi.org/10.1200/JCO.2009.23.7370
Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, Lang I, Ruhstaller T, Gianni L, Goldhirsch A, Kammler R, Price KN, Cancello G, Munzone E, Gelber RD, Regan MM, Colleoni M (2016) Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat 158(2):323–331. https://doi.org/10.1007/s10549-016-3863-3
Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N, Ishida T (2015) Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17:124. https://doi.org/10.1186/s13058-015-0632-x
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. https://doi.org/10.1126/science.1129139
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A (2016) Mutations Associated with acquired resistance to PD-1 Blockade in Melanoma. N Engl J Med 375(9):819–829. https://doi.org/10.1056/NEJMoa1604958
Konig L, Mairinger FD, Hoffmann O, Bittner AK, Schmid KW, Kimmig R, Kasimir-Bauer S, Bankfalvi A (2019) Dissimilar patterns of tumor-infiltrating immune cells at the invasive tumor front and tumor center are associated with response to neoadjuvant chemotherapy in primary breast cancer. BMC Cancer 19(1):120. https://doi.org/10.1186/s12885-019-5320-2
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, Kuroi K, Im SA, Park BW, Kim SB, Yanagita Y, Ohno S, Takao S, Aogi K, Iwata H, Jeong J, Kim A, Park KH, Sasano H, Ohashi Y, Toi M (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159. https://doi.org/10.1056/NEJMoa1612645
Swisher SK, Wu Y, Castaneda CA, Lyons GR, Yang F, Tapia C, Wang X, Casavilca SA, Bassett R, Castillo M, Sahin A, Mittendorf EA (2016) Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Ann Surg Oncol 23(7):2242–2248. https://doi.org/10.1245/s10434-016-5173-8
Mani NL, Schalper KA, Hatzis C, Saglam O, Tavassoli F, Butler M, Chagpar AB, Pusztai L, Rimm DL (2016) Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer. Breast Cancer Res 18(1):78. https://doi.org/10.1186/s13058-016-0737-x
Steele KE, Tan TH, Korn R, Dacosta K, Brown C, Kuziora M, Zimmermann J, Laffin B, Widmaier M, Rognoni L, Cardenes R, Schneider K, Boutrin A, Martin P, Zha J, Wiestler T (2018) Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer 6(1):20. https://doi.org/10.1186/s40425-018-0326-x
Klauschen F, Muller KR, Binder A, Bockmayr M, Hagele M, Seegerer P, Wienert S, Pruneri G, de Maria S, Badve S, Michiels S, Nielsen TO, Adams S, Savas P, Symmans F, Willis S, Gruosso T, Park M, Haibe-Kains B, Gallas B, Thompson AM, Cree I, Sotiriou C, Solinas C, Preusser M, Hewitt SM, Rimm D, Viale G, Loi S, Loibl S, Salgado R, Denkert C (2018) Scoring of tumor-infiltrating lymphocytes: From visual estimation to machine learning. Semin Cancer Biol 52(Pt 2):151–157. https://doi.org/10.1016/j.semcancer.2018.07.001
Kato T, Park JH, Kiyotani K, Ikeda Y, Miyoshi Y, Nakamura Y (2017) Integrated analysis of somatic mutations and immune microenvironment of multiple regions in breast cancers. Oncotarget 8(37):62029–62038. https://doi.org/10.18632/oncotarget.18790
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33(17):1974–1982. https://doi.org/10.1200/JCO.2014.59.4358
Acknowledgements
The authors would like to thank S. Haraguchi at the research center of Sagara Hospital for extracting the necessary information from the database, and the technicians at the Department of Pathology at Sagara Hospital for their assistance in dealing with archived samples. We thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This study was funded by the Division of Clinical Research Promotion at the National Hospital Organization Shikoku Cancer Center.
Author information
Authors and Affiliations
Contributions
AH, KA, and YO planned the study. AH and TW conducted pathological assessments. Yasuaki S., Yoshiaki S., and MK performed biopsies and/or surgeries, and provided oncological treatment. KA supervised the whole study. The manuscript was mainly written by AH, and amended by YO and AT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AH received personal fees as honoraria from Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. YS received personal fees as honoraria from AstraZeneca, Chugai Pharmaceutical, Pfizer, Eisai, Novartis Pharma, Taiho Pharmaceutical, and Takeda Pharmaceutical. MK received personal fees as honoraria from Chugai Pharmaceutical, Eisai, AstraZeneca, Pfizer, Taiho Pharmaceutical, Novartis Pharma, Takeda Pharmaceutical, Daiichi Sankyo, Kyowa Hakko Kirin, Shionogi, and Asashi Kasei. KA received personal fees as honoraria from Chugai Pharmaceutical, Eisai, AstraZeneca, Taiho Pharmaceutical, Novartis Pharma, Daiichi Sankyo, Mochida Pharmaceutical, Ono Pharmaceutical, and Eli Lilly Japan, and his institution received research funds from Chugai Pharmaceutical, Eisai and Sanofi. The other authors have no competing interests to declare.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee (No. 14-06 & 17-33) and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Formal consent was not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hida, A.I., Watanabe, T., Sagara, Y. et al. Diffuse distribution of tumor-infiltrating lymphocytes is a marker for better prognosis and chemotherapeutic effect in triple-negative breast cancer. Breast Cancer Res Treat 178, 283–294 (2019). https://doi.org/10.1007/s10549-019-05390-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05390-x