Abstract
Purpose
GEICAM/2006–10 compared anastrozole (A) versus fulvestrant plus anastrozole (A + F) to test the hypothesis of whether a complete oestrogen blockade is superior to aromatase inhibitors alone in breast cancer patients receiving hormone adjuvant therapy.
Methods
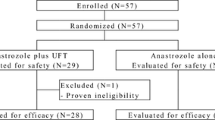
Multicenter, open label, phase III study. HR+/HER2− EBC postmenopausal patients were randomized 1:1 to adjuvant A (5 years [year]) or A + F (A plus F 250 mg/4 weeks for 3 year followed by 2 year of A). Stratification factors: prior chemotherapy (yes/no); number of positive lymph nodes (0/1–3/≥ 4); HR status (both positive/one positive) and site. Primary objective: disease-free survival (DFS). Planned sample size: 2852 patients.
Results
The study has an early stop due to the financer decision with 870 patients (437 randomized to A and 433 to A + F). Patient characteristics were well balanced. After a median follow-up of 6.24y and 111 DFS events (62 in A and 49 in A + F) the Hazard Ratio for DFS (combination vs. anastrozole) was 0.84 (95% CI 0.58–1.22; p = 0.352). The proportion of patients disease-free in arms A and A + F at 5 year and 7 year were 90.8% versus 91% and 83.6% versus 86.7%, respectively. Most relevant G2-4 toxicities (≥ 5% in either arm) with A versus A + F were joint pain (14.7%; 13.7%), fatigue (2.5%; 7.2%), bone pain (3%; 6.5%), hot flushes (3.5%; 5%) and muscle pain (2.8%; 5.1%).
Conclusions
The GEICAM/2006–10 study did not show a statistically significant increase in DFS by adding adjuvant F to A, though no firm conclusions can be drawn because of the limited sample size due to the early stop of the trial. ClinicalTrials.gov: NCT00543127.





Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group, Davies C, Godwin J et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784
Early Breast Cancer Trialists’ Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352
Garcia-Saenz JA, Bermejo B, Estevez LG et al (2015) SEOM clinical guidelines in early-stage breast cancer. Clin Transl Oncol 17:939–945
Burstein HJ, Prestrud AA, Seidenfeld J et al (2010) American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28:3784–3796
Senkus E, Kyriakides S, Ohno S et al (2015) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–30
Nathan MR, Schmid P (2017) A review of fulvestrant in breast cancer. Oncol Ther 5:17–29
Robertson JFR, Bondarenko IM, Trishkina E et al (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388:2997–3005
Bergh J, Jonsson PE, Lidbrink EK et al (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30:1919–1925
Mehta RS, Barlow WE, Albain KS et al (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367:435–444
Robertson JF, Dixon JM, Sibbering DM et al (2013) A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res 15:R18
Common Terminology Criteria for Adverse Events v3.0 (CTCAE) (2006)
Di Leo A, Jerusalem G, Petruzelka L et al (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28:4594–4600
Di Leo A, Jerusalem G, Petruzelka L et al (2014) Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 106:djt337
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62:233–247
Fernandez SV, Russo J (2010) Estrogen and xenoestrogens in breast cancer. Toxicol Pathol 38:110–122
Sestak I, Dowsett M, Zabaglo L et al (2013) Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 105:1504–1511
Negrouk A, Lacombe D, Cardoso F et al (2017) Safeguarding the future of independent, academic clinical cancer research in Europe for the benefit of patients. ESMO Open 2:e000187
Acknowledgements
We thank all the investigators involved in the GEICAM/2006–10 study (M.A. Seguí, F. Ayala, J. de la Haba, P. Martínez, S. González, A. Lahuerta, J.C. Toral, E. Martínez de Dueñas, J. Florián, M.J. Godes, C. Llorca, I. Blancas, C. Jara, S. Morales, A. Arcusa, A. Martínez, E. Vicente, A. de Juan, M. Rodríguez, M. García, P. García, J.L. Bayo, V. Carañana, J. Casinello, L. Jolis, M. Gil, C. Cañabate, A. Oltra, J. Ramírez, M. Lomas, A. Barnadas, M. Sureda, F. Carabantes, I. Moreno, A.L. Moreno). We also thank all of the participating patients and local research staff as well as the GEICAM staff.
Funding
The study was sponsored by GEICAM Spanish Breast Cancer Group. AstraZeneca Farmaceutica Spain provided fulvestrant and funding for the trial.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
B Bermejo her institution has received research funding from AstraZeneca, Merck, Roche Pharma AG, Boehringer Ingelheim, Novartis and Roche outside the current trial. M. Ramos has participated in advisory boards of AstraZeneca, Roche, Novartis and Pfizer and received speaker honoraria from AstraZeneca, Roche, Novartis and Pfizer. J. Cruz has participated in advisory boards of AstraZeneca, Roche, Novartis, Pharmamar, Eisai, Lilly, Celgene, Astellas, Amgen, Glaxo, and Pfizer and received speaker honoraria from Glaxo, AstraZeneca, Roche, Novartis, Pharmamar, Eisai, Lilly, Celgene, Astellas, Amgen, and Pfizer. J.M. Baena-Cañada has participated in advisory boards of AstraZeneca, Roche, Novartis, Amgen, Bristol, Eisay, Celgene and Pfizer and received speaker honoraria from Roche and Ferrer. His institution has received research funding from AstraZeneca. B. Cirauqui has received speaker honoraria and funding for some independent medical education activities from Astra Zeneca outside de current trial. A Rodríguez-Lescure has participated in advisory boards of Roche, Novartis, MSD and Pfizer and has received speaker honoraria from AstraZeneca, Roche, Novartis, Kern Pharma, Amgen, and Pfizer; his institution has received research funding from Novartis, Pfizer, Lilly and Roche outside the current trial. E. Alba has participated in advisory boards of Roche, Pfizer, Novartis and Lilly, his institution has received funding from Roche and Sysmex. N. Martínez Jañez has participated in advisory boards of AstraZeneca, Roche, Novartis, Celgene, Eisai, and Pfizer and received speaker honoraria from Roche, Novartis, Eisai and Pfizer. M. Muñoz: has participated in advisory boards of AstraZeneca, Merck and Novartis and expert opinion of Roche. She has received scientific meetings travel expenses from AstraZeneca and Roche. I. Álvarez López has participated in consultant or advisory boards of AstraZeneca, Pfizer, Palex, Roche, Novartis and received speaker honoraria from AstraZeneca, Pfizer, Roche, Eisai, received scientific meetings travel expenses from Pfizer and Roche. Her institution has received research funding from AstraZeneca, Pfizer, Roche, Novartis outside the current trial. A. Antón: has participated in consultant or advisory boards of Bayer. Him institution has received research funding from Roche outside the current trial. E. Carrasco´s immediate family member has participated in advisory boards of Pfizer and received speaker honoraria form Pfizer and his institution has received research funding from Pfizer. M. Martin has participated in advisory boards of AstraZeneca, Roche, Novartis, Pharmamar, Amgen, and Pfizer and received speaker honoraria from Glaxo, AstraZeneca, Roche, Novartis, Amgen, and Pfizer; his institution has received research funding from Novartis and Roche outside the current trial. E. Sevillano has received speaker honorary from Roche, Eisai, MSD and Ipsen. GEICAM has received funding from AstraZeneca for some independent medical education activities and research projects performed by the Group in which all authors collaborated. Rest of authors declare no conflict of interest.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki, approved by the institutions’ ethical committees and health authorities in Spain, and registered at EUDRACT (2007-003417-14) and ClinicalTrials.gov (NCT00543127). Written informed consent was obtained from all patients before performing any protocol specific procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of GEICAM participant investigators are listed in “Acknowledgement” section.
Rights and permissions
About this article
Cite this article
Ruíz-Borrego, M., Guerrero-Zotano, A., Bermejo, B. et al. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2−) early breast cancer (EBC): results from the GEICAM/2006–10 study. Breast Cancer Res Treat 177, 115–125 (2019). https://doi.org/10.1007/s10549-019-05296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05296-8