Abstract
Purpose
Tamoxifen is an important targeted endocrine therapy in breast cancer. However, side effects and early discontinuation of tamoxifen remains a barrier for obtaining the improved outcome benefits of long-term tamoxifen treatment. Biomarkers predictive of tamoxifen side effects remain unidentified. The objective of this prospective population-based study was to investigate the value of tamoxifen metabolite concentrations as biomarkers for side effects. A second objective was to assess the validity of discontinuation rates obtained through pharmacy records with the use of tamoxifen drug monitoring.
Methods
Longitudinal serum samples, patient-reported outcome measures and pharmacy records from 220 breast cancer patients were obtained over a 6-year period. Serum concentrations of tamoxifen metabolites were measured by LC–MS/MS. Associations between metabolite concentrations and side effects were analyzed by logistic regression and cross table analyses. To determine the validity of pharmacy records we compared longitudinal tamoxifen concentrations to discontinuation rates obtained through the Norwegian Prescription database (NorPD). Multivariable Cox regression models were performed to identify predictors of discontinuation.
Results
At the 2nd year of follow-up, a significant association between vaginal dryness and high concentrations of tamoxifen, Z-4′-OHtam and tam-NoX was identified. NorPD showed a tamoxifen-discontinuation rate of 17.9% at 5 years and drug monitoring demonstrated similar rates. Nausea, vaginal dryness and chemotherapy-naive status were significant risk factors for tamoxifen discontinuation.
Conclusions
This real-world data study suggests that measurements of tamoxifen metabolite concentrations may be predictive of vaginal dryness in breast cancer patients and verifies NorPD as a reliable source of adherence data.


Similar content being viewed by others
References
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM et al (2005) Tamoxifen for the prevention of breast cancer: current status of the national surgical adjuvant breast and bowel project P-1 study. J Nat Cancer Inst 97(22):1652–1662. https://doi.org/10.1093/jnci/dji372
Jackisch C, Harbeck N, Huober J, von Minckwitz G, Gerber B et al (2015) 14th St. Gallen International Breast Cancer Conference 2015: evidence, controversies, consensus - primary therapy of early breast cancer: opinions expressed by German experts. Breast Care 10(3):211–219. https://doi.org/10.1159/000433590
Davies C, Pan H, Godwin J, Gray R, Arriagada R et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816
Gray RG, Rea D, Handley K, Bowden SJ, Perry P et al (2013) aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. https://doi.org/10.1200/jco.2013.31.18_suppl.5
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A et al (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128. https://doi.org/10.1200/jco.2009.25.9655
Owusu C, Buist DSM, Field TS, Lash TL, Thwin SS et al (2008) Predictors of tamoxifen discontinuation among older women with estrogen receptor–positive breast cancer. J Clin Oncol 26(4):549–555. https://doi.org/10.1200/jco.2006.10.1022
Aiello Bowles EJ, Boudreau DM, Chubak J, Yu O, Fujii M et al (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8(6):e149–e157. https://doi.org/10.1200/jop.2012.000543
Lorizio W, Wu AHB, Beattie MS, Rugo H, Tchu S et al (2012) Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat 132(3):1107–1118. https://doi.org/10.1007/s10549-011-1893-4
Garreau JR, DeLaMelena T, Walts D, Karamlou K, Johnson N (2006) Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg 192(4):496–498. https://doi.org/10.1016/j.amjsurg.2006.06.018
Ganz PA (2001) Impact of tamoxifen adjuvant therapy on symptoms, functioning, and quality of life. JNCI Monog 2001(30):130–134. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003450
Early Breast Cancer Trialists’ Collaborative G (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
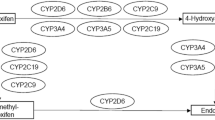
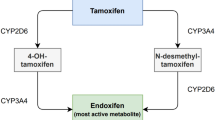
Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB et al (2013) PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics 23(11):643–647. https://doi.org/10.1097/FPC.0b013e3283656bc1
Coller JK, Krebsfaenger N, Klein K, Endrizzi K, Wolbold R et al (2002) The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol 54(2):157–167
Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE et al (2007) Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol 19(1):56–61. https://doi.org/10.1093/annonc/mdm434
Helland T, Henne N, Bifulco E, Naume B, Borgen E et al (2017) Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res 19:125. https://doi.org/10.1186/s13058-017-0916-4
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Therap 89(5):718–725. https://doi.org/10.1038/clpt.2011.32
Saladores P, Murdter T, Eccles D, Chowbay B, Zgheib NK et al (2015) Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 15(1):84–94. https://doi.org/10.1038/tpj.2014.34
Gm P, Katrin S, Henk-Jan G, Matthias S, Michael P et al (2018) Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Therap 103(5):770–777. https://doi.org/10.1002/cpt.1007
Jager NG, Linn SC, Schellens JH, Beijnen JH (2015) Tailored tamoxifen treatment for breast cancer patients: a perspective. Clin Breast Cancer 15(4):241–244
Barginear MF, Jaremko M, Peter I, Yu C, Kasai Y et al (2011) Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Therap 90(4):605–611. https://doi.org/10.1038/clpt.2011.153
Jr WJI, Walko CM, Weck KE, Ibrahim JG, Chiu WK et al (2011) Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J Clin Oncol 29(24):3232–3239. https://doi.org/10.1200/jco.2010.31.4427
Goetz MP, Suman VJ, Reid JM, Northfelt DW, Mahr MA et al (2017) First-in-human phase I study of the tamoxifen metabolite Z-endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol 2017(2073):3246
Gallicchio L, Lord G, Tkaczuk K, Danton M, Lewis LM et al (2004) Association of tamoxifen (TAM) and TAM metabolite concentrations with self-reported side effects of TAM in women with breast cancer. Breast Cancer Res Treat 85(1):89–97. https://doi.org/10.1023/B:BREA.0000021050.92539.b0
Peyrade F, Frenay M, Etienne M-C, Ruch F, Guillemare C et al (1996) Age-related difference in tamoxifen disposition. Clin Pharmacol Therap 59(4):401–410. https://doi.org/10.1016/S0009-9236(96)90108-3
Lunde S, Helland T, Jonassen J, Haugstøyl ME, Austdal M et al (2018) A prospective, longitudinal, breast cancer biobank (PBCB) in western Norway. Research Gate. https://doi.org/10.13140/rg.2.2.16266.11201
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55(2):189–199
Furu K (2008) Establishment of the nationwide Norwegian Prescription Database (NorPD)–new opportunities for research in pharmacoepidemiology in Norway. Norsk Epidemiologi. https://doi.org/10.5324/nje.v18i2.23
Ayres LR, Baldoni AdO, Borges APdS, Pereira LRL (2014) Adherence and discontinuation of oral hormonal therapy in patients with hormone receptor positive breast cancer. Int J Clin Pharm 36(1):45–54. https://doi.org/10.1007/s11096-013-9833-5
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606. https://doi.org/10.1200/JCO.2003.07.071
NBCG (2017) National action plan with guidelines for diagnosis, treatment and follow up of breast cancer patients. vol IS-2669. Norwegian Directory of Health, www.helsedirektoratet.no
Mom CH, Buijs C, Willemse PH, Mourits MJ, de Vries EG (2006) Hot flushes in breast cancer patients. Crit Rev Oncol/Hematol 57(1):63–77. https://doi.org/10.1016/j.critrevonc.2005.04.009
Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL et al (1996) Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Nat Cancer Inst 88(21):1529–1542. https://doi.org/10.1093/jnci/88.21.1529
Jager NG, Koornstra RH, Vincent AD, van Schaik RH, Huitema AD et al (2013) Hot flashes are not predictive for serum concentrations of tamoxifen and its metabolites. BMC Cancer 13:612. https://doi.org/10.1186/1471-2407-13-612
Polin SA, Ascher SM (2008) The effect of tamoxifen on the genital tract. Cancer Imaging 8(1):135–145. https://doi.org/10.1102/1470-7330.2008.0020
Tsuda K, Nishio I (2005) A selective estrogen receptor modulator, tamoxifen, and membrane fluidity of erythrocytes in normotensive and hypertensive postmenopausal women: an electron paramagnetic resonance investigation. Am J Hypertens 18(8):1067–1076. https://doi.org/10.1016/j.amjhyper.2005.02.009
Suwalsky M, Hernandez P, Villena F, Aguilar F, Sotomayor CP (1998) Interaction of the anticancer drug tamoxifen with the human erythrocyte membrane and molecular models. Zeitschrift fur Naturforschung C. J Biosci 53(3–4):182–190
Huiart L, Ferdynus C, Giorgi R (2013) A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat 138(1):325–328. https://doi.org/10.1007/s10549-013-2422-4
Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS (2006) Validity of a prescription claims database to estimate medication adherence in older persons. Med Care 44(5):471–477. https://doi.org/10.1097/01.mlr.0000207817.32496.cb
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the 5-year course. Breast Cancer Res Treat 99(2):215–220. https://doi.org/10.1007/s10549-006-9193-0
Demissie S, Silliman RA, Lash TL (2001) Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19(2):322–328. https://doi.org/10.1200/jco.2001.19.2.322
Lambertini M, Del Mastro L, Viglietti G, Ponde NF, Solinas C et al (2017) Ovarian function suppression in premenopausal women with early-stage breast cancer. Curr Treat Options Oncol 18(1):4. https://doi.org/10.1007/s11864-017-0442-8
Huiart L, Dell’Aniello S, Suissa S (2011) Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer 104(10):1558–1563. https://doi.org/10.1038/bjc.2011.140
Acknowledgements
We would like to thank all the patients that currently are, or have been, participating in the Prospective Breast Cancer Biobank (PBCB) project.
Funding
The present study was funded by the Western Norway Regional Health Authority, Folke Hermansen Foundation and Inge Steenslands Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The Prospective Breast Cancer Biobank in which this population was obtained was approved by the Norwegian Regional Ethical Committee (2010/1957 and 2011/2161).
Informed consent
All participants provided written informed consent before enrolling. All personal identifiers for each case in this population cohort were removed before analyses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Helland, T., Hagen, K.B., Haugstøyl, M.E. et al. Drug monitoring of tamoxifen metabolites predicts vaginal dryness and verifies a low discontinuation rate from the Norwegian Prescription Database. Breast Cancer Res Treat 177, 185–195 (2019). https://doi.org/10.1007/s10549-019-05294-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05294-w