Abstract
Purpose
We sought to identify trends over time with respect to the use of hypofractionated whole breast irradiation (HF-WBI) in women with triple negative breast cancer (TNBC) in the national cancer database (NCDB).
Methods
Trends in utilization of HF-WBI in women diagnosed with T1-2N0 TNBC in the NCDB between 2008 and 2013 were analyzed. Case-matched luminal A women were used for comparison. Variables included age, race, year of diagnosis, insurance status, income quartile, receipt of neoadjuvant chemotherapy, and institution (academic vs. community). Chi square, logistic regression, and multivariate analysis was performed.
Results
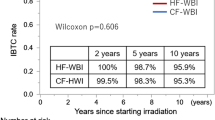
Utilization of HF-WBI among the 53,269 TNBC women identified steadily increased from 4.7% in 2008 to 14.0% in 2013 for women with TNBC compared to luminal A cancer whose utilization increased from 7.3 to 23.3% over the same time frame (p < 0.001). On univariate analysis, HF-WBI was associated with increasing age (p < 0.001), Medicare insurance (p < 0.001), race (p = 0.041), diagnosis after 2011 (p < 0.001), higher income quartile (p < 0.001), and treatment at academic institutions (p < 0.001). On multivariate analysis, age (p < 0.001, OR 1.038 per year), income quartile (p = 0.002, OR 1.061 per increase in quartile), treatment at an academic institution (p < 0.001, OR 1.78) significantly increased use of HF-WBI.
Conclusions
Treatment at an academic center and year of diagnosis were most correlated with increased HF-WBI in T1-2N0 TNBC women in the NCDB from 2008 to 2013, followed by increasing age and income. Only 14% of T1-2N0 TNBC women received HF-WBI in 2013. Focus on increased utilization is needed for non-academic centers, lower income, and younger women.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics (2018) CA Cancer J Clin 2018;68(1):7–30. https://doi.org/10.3322/caac.21442
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26(15):2568–2581. https://doi.org/10.1200/JCO.2007.13.1748
Reis-Filho JS, Tutt ANJ (2008) Triple negative tumours: a critical review. Histopathology 52(1):108–118. https://doi.org/10.1111/j.1365-2559.2007.02889.x
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948. https://doi.org/10.1056/NEJMra1001389
Trivers KF, Lund MJ, Porter PL et al (2009) The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 20(7):1071–1082. https://doi.org/10.1007/s10552-009-9331-1
Lin NU, Vanderplas A, Hughes ME et al (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118(22):5463–5472. https://doi.org/10.1002/cncr.27581
Gonzalez-Angulo AM, Timms KM, Liu S et al (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 17(5):1082–1089. https://doi.org/10.1158/1078-0432.CCR-10-2560
Colleoni M, Cole BF, Viale G et al (2010) Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol 28(18):2966–2973. https://doi.org/10.1200/JCO.2009.25.9549
Isakoff SJ, Mayer EL, He L et al (2015) TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol 33(17):1902–1909. https://doi.org/10.1200/JCO.2014.57.6660
Silver DP, Richardson AL, Eklund AC et al (2010) Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153. https://doi.org/10.1200/JCO.2009.22.4725
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15):4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045
Trialists TS (2008) The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 9(4):331–341. https://doi.org/10.1016/S1470-2045(08)70077-9
Haviland JS, Owen JR, Dewar JA et al (2013) The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 14(11):1086–1094. https://doi.org/10.1016/S1470-2045(13)70386-3
Agrawal RK, Aird EGA, Barrett JM et al (2008) The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371(9618):1098–1107. https://doi.org/10.1016/S0140-6736(08)60348-7
Yarnold J, Ashton A, Bliss J et al (2005) Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 75(1):9–17. https://doi.org/10.1016/j.radonc.2005.01.005
Owen JR, Ashton A, Bliss JM et al (2006) Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 7(6):467–471. https://doi.org/10.1016/S1470-2045(06)70699-4
Whelan TJ, Pignol J-P, Levine MN et al (2010) Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362(6):513–520. https://doi.org/10.1056/NEJMoa0906260
Smith BD, Bentzen SM, Correa CR et al (2011) Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol 81(1):59–68. https://doi.org/10.1016/j.ijrobp.2010.04.042
Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC (2014) Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys 90(5):1001–1009. https://doi.org/10.1016/j.ijrobp.2014.09.032
Jagsi R, Griffith KA, Heimburger D et al (2014) Choosing wisely? Patterns and correlates of the use of hypofractionated whole-breast radiation therapy in the state of Michigan. Int J Radiat Oncol Biol Phys 90(5):1010–1016. https://doi.org/10.1016/j.ijrobp.2014.09.027
Gilbo P, Potters L, Lee L (2018) Implementation and utilization of hypofractionation for breast cancer. Adv Radiat Oncol 3(3):265–270. https://doi.org/10.1016/j.adro.2018.04.001
Bane AL, Whelan TJ, Pond GR et al (2014) Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol 25(5):992–998. https://doi.org/10.1093/annonc/mdu090
Smith BD, Bellon JR, Blitzblau R et al (2018) Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 8(3):145–152. https://doi.org/10.1016/j.prro.2018.01.012
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National cancer data base: a powerful initiative to improve cancer care in the united states. Ann Surg Oncol 15(3):683–690. https://doi.org/10.1245/s10434-007-9747-3
Bekelman JE, Sylwestrzak G, Barron J et al (2014) Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA 312(23):2542. https://doi.org/10.1001/jama.2014.16616
Shaitelman SF, Schlembach PJ, Arzu I et al (2015) Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol 1(7):931–941. https://doi.org/10.1001/jamaoncol.2015.2666
Vargas L, Solé S, Solé CV (2018) Cosmesis after early stage breast cancer treatment with surgery and radiation therapy: experience of patients treated in a Chilean radiotherapy centre. Ecancermedicalscience 12:819. https://doi.org/10.3332/ecancer.2018.819
Taher AN, El-Baradie MM, Essa H, Zaki O, Ezzat S (2004) Hypofractionation versus conventional fractionation radiotherapy after conservative treatment of breast cancer: early skin reactions and cosmetic results. J Egypt Natl Canc Inst 16(3):178–187
Funding
No outside funding was used in the preparation, design, or writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rice, S.R., Feigenberg, S.J., Hamza, M. et al. Trends in utilization of hypofractionated whole breast irradiation (HF-WBI) in triple negative breast cancer (TNBC): a national cancer database (NCDB) analysis. Breast Cancer Res Treat 175, 473–478 (2019). https://doi.org/10.1007/s10549-019-05150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05150-x