Abstract
Purpose
There is an urgent need for the development of a predictor of response to chemotherapy for ER-positive breast cancer which is less chemosensitive than for ER-negative breast cancer in order to avoid unnecessary chemotherapy. In the present study, intrinsic subtyping by PAM50 was evaluated for its ability to predict a response to chemotherapy.
Patients and Methods
For this study, 124 patients with ER-positive breast cancer treated with neoadjuvant sequential paclitaxel and FEC (NAC) were evaluated. Tumor biopsy specimens obtained before NAC were subjected to intrinsic subtyping (IS) by gene expression (GE) using PAM50 (PAM50-IS) or immunohistochemistry (IHC-IS).
Results
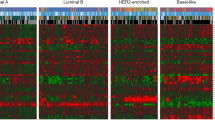
Of the PAM50-ISs (Luminal A, Luminal B, HER2-enriched, and Basal-like), GE-Luminal A showed the lowest pCR rate (1.9%), and multivariate analysis revealed that GE-Luminal A was a significant (P = 0.031) predictor of non-pCR independently of other clinicopathological parameters, including Ki67, and tumor-infiltrating lymphocytes. Of the IHC-ISs, on the other hand, IHC-Luminal A was not significantly associated with pCR. We also found that breast tumors with low ER levels (1–9%), like ER-negative tumors, were mostly GE-HER2-enriched and GE-Basal-like, and more sensitive to NAC than those with high ER levels (≥ 10%).
Conclusions
GE-Luminal A intrinsically subtyped by PAM50 was the least sensitive to NAC and very unlikely to attain pCR. IHC-Luminal A identified by IHC, on the other hand, was not significantly predictive of pCR. In addition, PAM50 revealed that tumors with low ER (1–9%) were more like ER-negative tumors than ER-positive tumors, and most such cases should therefore would better be treated with chemotherapy.




Similar content being viewed by others
Abbreviations
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- DMFS:
-
Distant metastasis-free survival
- pCR:
-
Pathological complete response
- IHC:
-
Immunohistochemistry
- FISH:
-
Fluorescence in situ hybridization
- HG:
-
Histological grade
- NAC:
-
Neoadjuvant chemotherapy
- GE:
-
Gene expression
- IS:
-
Intrinsic subtype
References
Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J, Moldovan C et al (2014) Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1 – 00 phase III trial. Ann Oncol 25(6):1128–1136. https://doi.org/10.1093/annonc/mdu118
Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, Blum RH (2006) Primary systemic therapy of breast cancer. Oncologist 11(6):574–589. https://doi.org/10.1634/theoncologist.11-6-574
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L et al (2006) The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genom 7:96. https://doi.org/10.1186/1471-2164-7-96
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z et al (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167. https://doi.org/10.1200/JCO.2008.18.1370
Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, Ferree S, Storhoff J, Schaper C, Cuzick J (2013) Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 31(22):2783–2790. https://doi.org/10.1200/JCO.2012.46.1558
Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, Mlineritsch B, Kwasny W, Knauer M, Singer C et al (2014) Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 25(2):339–345. https://doi.org/10.1093/annonc/mdt494
Prat A, Fan C, Fernandez A, Hoadley KA, Martinello R, Vidal M, Viladot M, Pineda E, Arance A, Munoz M et al (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13:303. https://doi.org/10.1186/s12916-015-0540-z
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel m (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Nakauchi C, Naoi Y, Shimazu K, Tsunashima R, Nishio M, Maruyama N, Shimomura A, Kagara N, Shimoda M, Kim SJ et al (2014) Development of a prediction model for lymph node metastasis in luminal A subtype breast cancer: the possibility to omit sentinel lymph node biopsy. Cancer Lett 353(1):52–58. https://doi.org/10.1016/j.canlet.2014.07.003
Sota Y, Naoi Y, Tsunashima R, Kagara N, Shimazu K, Maruyama N, Shimomura A, Shimoda M, Kishi K, Baba Y et al (2014) Construction of novel immune-related signature for prediction of pathological complete response to neoadjuvant chemotherapy in human breast cancer. Ann Oncol 25(1):100–106. https://doi.org/10.1093/annonc/mdt427
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422. https://doi.org/10.1200/JCO.2007.10.6823
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664. https://doi.org/10.1093/jnci/djr393
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Lee JK, Coutant C, Kim YC, Qi Y, Theodorescu D, Symmans WF, Baggerly K, Rouzier R, Pusztai L (2010) Prospective comparison of clinical and genomic multivariate predictors of response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res 16(2):711–718. https://doi.org/10.1158/1078-0432.CCR-09-2247
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750. https://doi.org/10.1093/jnci/djp082
Viale G, de Snoo FA, Slaets L, Bogaerts J, van ‘t Veer L, Rutgers EJ, Piccart-Gebhart MJ, Stork-Sloots L, Glas A, Russo L et al (2018) Immunohistochemical versus molecular (BluePrint and MammaPrint) subtyping of breast carcinoma. Outcome results from the EORTC 10041/BIG 3–04 MINDACT trial. Breast Cancer Res Treat 167(1):123–131. https://doi.org/10.1007/s10549-017-4509-9
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol 29(17):2342–2349. https://doi.org/10.1200/jco.2010.31.6950
Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J et al (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16(21):5222–5232. https://doi.org/10.1158/1078-0432.ccr-10-1282
Suman VJ, Ellis MJ, Ma CX: The ALTERNATE trial (2015) assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 4(3):34. https://doi.org/10.3978/j.issn.2304-3865.2015.09.01
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr et al (2018) Adjuvant Chemotherapy guided by a 21-Gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M et al (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729. https://doi.org/10.1056/NEJMoa1602253
Scott D, Ramsey WE, Barlow AM, Gonzalez-Angulo S, Tunis L, Baker J, Crowley P, Deverka D, Veenstra D, Hortobagyi GN (2013) Integrating comparative effectiveness design elements and endpoints into a phase iii, randomized clinical trial (SWOG S1007) evaluating oncotypedx-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials 34(1):1–9. https://doi.org/10.1016/j.cct.2012.09.003
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y et al (2012) Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1–10% ER-positive by immunohistochemistry. J Clin Oncol 30(7):729–734. https://doi.org/10.1200/JCO.2011.36.2574
Fujii T, Kogawa T, Dong W, Sahin AA, Moulder S, Litton JK, Tripathy D, Iwamoto T, Hunt KK, Pusztai L et al (2017) Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol 28(10):2420–2428. https://doi.org/10.1093/annonc/mdx397
Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, Bedrosian I, Buzdar AU, Symmans WF, Crow JR et al (2014) Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol 25(5):1004–1011. https://doi.org/10.1093/annonc/mdu053
Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q (2018) Borderline ER-positive primary breast Cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer 18(1):1–8. https://doi.org/10.1016/j.clbc.2017.06.005
Raghav KP, Hernandez-Aya LF, Lei X, Chavez-Macgregor M, Meric-Bernstam F, Buchholz TA, Sahin A, Do KA, Hortobagyi GN, Gonzalez-Angulo AM (2012) Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer 118(6):1498–1506. https://doi.org/10.1002/cncr.26431
Acknowledgements
This study was supported, in part, by the Knowledge Cluster Initiative of the Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Shinzaburo Noguchi has been an advisor for Taiho, AstraZeneca and Novartis, and has received research funding for other studies from Sysmex, AstraZeneca, Novartis, Chugai, Daiichi-Sankyo, Kyowa-Kirin, Takeda, Pfizer, Ono, Taiho, and Eisai, and honoraria from AstraZeneca, Novartis, Pfizer, Chugai, Takeda, Sysmex, Nippon Kayaku, and Ono. Dr. Yasuto Naoi has received research funding from Sysmex and AstraZeneca. Dr. Naofumi Kagara has received honoraria from AstraZeneca and Novartis. Dr. Masafumi Shimoda has received research funding from Novartis and AstraZeneca, and honoraria from Chugai, Eisai, Novartis, and Takeda. Dr. Kenzo Shimazu has received honoraria from AstraZeneca, Chugai, and Sysmex. Dr. Seung Jin Kim received honoraria from AstraZeneca, Chugai, Eisai, Kyowa-Kirin, Novartis, Pfizer, Shimadzu, Taiho, and Takeda.
Ethical approal
This study complies with the current relevant laws of and guidelines for Japan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2018_5020_MOESM1_ESM.docx
Supplementary Fig.1. Comparison of DMFS between ER-negative, ER-low, and ER-high breast cancers. Patients (n = 32) with ER-negative (0%) tumors were treated with neoadjuvant chemotherapy and those with ER-low (1–9%) tumors (n = 16) or ER-high (> 10%) tumors (n = 108) were treated with neoadjuvant chemotherapy plus adjuvant hormonal therapy. (DOCX 17 KB)
Rights and permissions
About this article
Cite this article
Ohara, A.M., Naoi, Y., Shimazu, K. et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat 173, 533–543 (2019). https://doi.org/10.1007/s10549-018-5020-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-5020-7