Abstract
Purpose
Therapeutic exploitation of angiogenesis in breast cancer has been limited by the lack of reliable biomarkers. Circulating small-sized endothelial microparticles (sEMP) are likely to play a significant role as messengers of angiogenesis. Higher levels of EMP have been observed in cancer patients, but their prognostic value in breast cancer is unknown. Our aim was to determine the value of circulating sEMP as a marker of response to chemotherapy in breast cancer.
Methods
We included patients with breast cancer treated with neoadjuvant or first-line chemotherapy. Baseline and post-treatment circulating sEMP (CD144+) were quantified using a flow cytometer approach specifically designed for analysis of small-sized particles (0.1–0.5 μm). Small-sized EMP response was defined as a post-treatment decrease of sEMP larger than the median decrease of sEMP after chemotherapy. Baseline and post-chemotherapy VEGFA levels were determined with ELISA.
Results
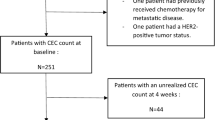
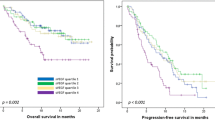
Forty-four breast cancer patients were included (19 with metastatic and 25 with locally advanced disease). Median levels of sEMP decreased after chemotherapy (P = 0.005). Response to chemotherapy showed a non-significant trend to associate with sEMP response (P = 0.056). A sEMP response was observed in 51% of patients and was associated with better overall survival (HR 0.18; 95% CI 0.04–0.87; P = 0.02) and progression free survival (HR 0.30; 95% CI 0.09–0.99; P = 0.04) in the group of women with metastatic disease. Post-chemotherapy decrease of VEGFA levels was not associated with breast cancer prognosis.
Conclusions
Our results did not support sEMP as a marker of response to chemotherapy. However, our exploratory analysis suggests that in patients with metastatic breast cancer, the decrease of sEMP levels after chemotherapy is associated with better overall and disease free survival and might be superior to VEGFA levels as an angiogenesis-related prognostic marker.


Similar content being viewed by others
Abbreviations
- BC:
-
Breast cancer
- CT:
-
Chemotherapy
- HR:
-
Hormonal receptors
- LABC:
-
Locally advanced breast cancer
- MBC:
-
Metastatic breast cancer
- MP:
-
Microparticles
- MV:
-
Extracellular microvesicles
- sEMP:
-
Small-sized endothelial microparticles
- TNBC:
-
Triple negative breast cancer
- VEGFA:
-
Vascular and endothelial growth factor A
References
Miles DW, Diéras V, Cortés J et al (2013) First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol 24:2773–2780
Ghosh S, Sullivan CAW, Zerkowski MP et al (2008) High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol 39:1835–1843
Berns EMJJ, Klijn JGM, Look MP et al (2003) Combined vascular endothelial growth factor and TP53 status predicts poor response to tamoxifen therapy in estrogen receptor-positive advanced breast cancer. Clin Cancer Res 9:1253–1258
Foekens JA, Peters HA, Grebenchtchikov N et al (2001) High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res 61:5407–5414
Lissoni P, Rovelli F, Malugani F et al (2003) Changes in circulating VEGF levels in relation to clinical response during chemotherapy for metastatic cancer. Int J Biol Markers 18:152–155
dos Santos LV, Cruz MR, de Lopes G, Lima JP (2015) VEGF-A levels in bevacizumab-treated breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat 151:481–489
Al-Nedawi K, Rak J, D’Asti E et al (2011) Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular “debris”. Semin Immunopathol 33:455–467
Lozito TP, Tuan RS (2014) Endothelial and cancer cells interact with mesenchymal stem cells via both microparticles and secreted factors. J Cell Mol Med 18:2372–2384
Piccin A, Murphy WG, Smith OP (2007) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21:157–171
Orozco AF, Lewis DE (2010) Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 77:502–514
Melo SA, Luecke LB, Kahlert C et al (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523:177–182
Valenti R, Huber V, Iero M et al (2007) Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 67:2912–2915
Martinez MC, Andriantsitohaina R (2011) Microparticles in Angiogenesis: therapeutic Potential. Circ Res 109:110–119
Ribeiro MF, Zhu H, Millard RW, Fan G-C (2013) Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes 2:54–59
Taraboletti G, D’Ascenzo S, Borsotti P et al (2002) Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160:673–680
Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ (2006) Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion 46:1199–1209
Millimaggi D, Mari M, D’Ascenzo S et al (2007) Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 9:349–357
Hoyer FF, Nickenig G, Werner N (2010) Microparticles–messengers of biological information. J Cell Mol Med 14:2250–2256
Sheremata WA, Jy W, Delgado S et al (2006) Interferon-beta1a reduces plasma CD31 + endothelial microparticles (CD31 + EMP) in multiple sclerosis. J Neuroinflammation 3:23
Montoro-García S, Shantsila E, Tapp LD et al (2013) Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 227:313–322
Tseng C-C, Wang C-C, Chang H-C et al (2013) Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis Markers 35:301–310
Laresche C, Pelletier F, Garnache-Ottou F et al (2013) Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma. J Invest Dermatol 134:176–182
Wang C-C, Tseng C-C, Hsiao C-C et al (2014) Circulating endothelial-derived activated microparticle: a useful biomarker for predicting 1-year mortality in patients with advanced non-small cell lung cancer. Biomed Res Int 2014:173401
Campello E, Spiezia L, Radu CM et al (2011) Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res 127:473–477
Toth B, Nieuwland R, Liebhardt S et al (2008) Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res 28:1107–1112
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
McShane LM, Altman DG, Sauerbrei W et al (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Montoro-García S, Shantsila E, Orenes-Piñero E et al (2012) An innovative flow cytometric approach for small-size platelet microparticles: influence of calcium. Thromb Haemost 108:373–383
Aharon A, Sabbah A, Ben-shaul S et al (2017) Chemotherapy administration to breast cancer patients affects extracellular vesicles thrombogenicity and function. Oncotarget 8:63265–63280
Shantsila E, Montoro-García S, Gallego P, Lip GYH (2014) Circulating microparticles: challenges and perspectives of flow cytometric assessment. Thromb Haemost 111:1009–1014
Sabatier F, Camoin-Jau L, Anfosso F et al (2009) Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med 13:454–471
Lechner D, Kollars M, Gleiss A et al (2007) Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost 5:2445–2452
Bovy N, Blomme B, Frères P et al (2015) Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget 6:10253–10266
Andre N, Cointe S, Barlogis V, Arnaud L et al (2015) Maintenance chemotherapy in children with aLL exerts metronomic-like thrombospondin-1 associated anti-endothelial effect. Oncotarget 6:23008–23014
Tang J-H, Zhao J-H, Lu J-W et al (2011) Circulating levels of angiogenic cytokines in advanced breast cancer patients with system chemotherapy and their potential value in monitoring disease course. J Cancer Res Clin Oncol 137:55–63
Munster M, Fremder E, Miller V et al (2014) Anti-VEGF-A affects the angiogenic properties of tumor-derived microparticles. PLoS ONE 9:e95983. https://doi.org/10.1371/journal.pone.0095983
Acknowledgements
We acknowledge technical assistance by José A. López Oliva (Clinical Research Unit, Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer) and Alberto Martínez (Biobancmur, Nodo 3, Hospital Universitario Morales Meseguer, IMIB Arrixaca). This work was partially funded by “Calasparra se mueve,” a research-funding initiative inspired by women from Calasparra (Murcia), Spain. SMG was supported by the People Program (Marie Curie Actions) of the European Union’s Seventh Framework Program (FP7/2007-2013) under REA Grant Agreement No. 608765.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study and the Institutional Review Board of Hospital Morales Meseguer approved the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García Garre, E., Luengo Gil, G., Montoro García, S. et al. Circulating small-sized endothelial microparticles as predictors of clinical outcome after chemotherapy for breast cancer: an exploratory analysis. Breast Cancer Res Treat 169, 83–92 (2018). https://doi.org/10.1007/s10549-017-4656-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4656-z