Abstract
Purpose
The practice of seeking a biopsy to confirm a metastatic relapse of a prior breast cancer is individualized. Tumor samples have well-recognized importance in clinical and translational research, but also an increasing role in routine care. We sought to determine the attitudes of patients and breast cancer clinicians about biopsy at breast cancer relapses.
Methods
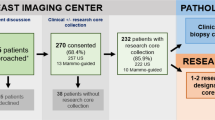
Consenting breast cancer patients and clinicians completed questionnaires with scenarios of decreasing personal benefit and increasing discomfort or inconvenience associated with biopsy at relapse of a prior breast cancer. For each scenario, patients were asked whether they would, would not, or were unsure about agreeing to a biopsy. Clinicians provided information about their practice, research activities, and usual biopsy habits. They were asked to estimate how often patients would agree to a biopsy under each of the conditions presented to patient participants.
Results
The majority of patients expressed a willingness to undergo a biopsy procedure of modest inconvenience and discomfort to establish an uncertain diagnosis, guide treatment, to participate in a trial, or for research purposes only. About 50% of patients indicated that they would undergo an invasive biopsy procedure requiring IV sedation or general anesthetic for purely altruistic reasons. In spite of being a largely academic group, clinician respondents underestimated patient willingness to have a biopsy in all scenarios, particularly when there was no attached personal benefit.
Conclusion
Breast cancer patients were very willing to undergo biopsy at breast cancer relapse for their routine care, clinical trials, or for research only. Clinicians act as the intermediary between patients and tumor tissue repositories, and clinician perceptions and practices should shift to match the altruistic attitudes of breast cancer patients.



Similar content being viewed by others
References
Peppercorn J, Shapira I, Collyar D et al (2010) Ethics of mandatory research biopsy for correlative endpoints within clinical trials in oncology. J Clin Oncol 28:2635–2640
Thompson AM, Jordan LB, Quinlan P et al (2010) Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the breast recurrence in tissues study (BRITS). Breast Cancer Res. https://doi.org/10.1186/bcr2771/12:R92
Broom RJ, Tang PA, Simmons C et al (2009) Changes in estrogen receptor, progesterone receptor and HER-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res 29:1557–1562
Amir E, Clemons M, Purdie CA et al (2012) Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev 38:708–714
Kuukasjarvi T, Karhu R, Tanner M et al (1997) Genetic heterogeneity and clonal evolution underlying development of asynchronous metastases in human breast cancer. Cancer Res 57:1597–1604
Aparicio S, Caldas C (2013) The implications of clonal evolution for cancer medicine. N Engl J Med 368:842–851
Vaught J, Rogers J, Myers K et al (2011) An NCI perspective on creating sustainable biospecimen resources. JNCI Monogr 42:1–7
William R, Watson G, Kay EW, Smith D (2010) Integrating biobanks: addressing the practical and ethical issues to deliver a valuable tool for cancer research. Nat Rev Cancer 10:646–651
Riegman DHJ, De Jong BWD, Llombart-Bosch A (2010) The organization of European Cancer Institute pathobiology working group and its support of European biobanking infrastructures for translational cancer research. Cancer Epidemiol Biomark Prev 19:923–926
Cottin V, Arpin D, Lasset C et al (1999) Small-cell lung cancer: patients included in clinical trials are not representative of the patient population as a whole. Ann Oncol 10:809–815
Murthy VH, Krumholz HM, Gross CP (2004) Participation in cancer clinical trials race, sex, and age based disparities. JAMA 291:2720–2726. https://doi.org/10.1001/jama.291.22.2720
Truong TH, Weeks JC, Cook EF, Joffe S (2011) Altruism among participants in cancer clinical trials. Clin Trials 8:616–623
Todd AMH, Laird BJA, Boyle D et al (2009) A systematic review examining the literature on attitudes of patients with advanced cancer toward research. J Pain Symptom Manage 37:1078–1085
Seah DS, Scott SM, Najita J et al (2013) Attitudes of patients with metastatic breast cancer towards research biopsies. Ann Oncol 24:1853–1859
Seah DSE, Scott S, Guo J et al (2014) Attitudes of medical oncologists towards research biopsies in patients with newly diagnosed stage I–III breast cancer not enrolled in a clinical trial. Cancer Res. https://doi.org/10.1158/1538-7445
Simmons C, Miller N, Geddie W et al (2009) Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol 20:1499–1504
Jenkings V, Fallowfield L (2000) Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. BJC 82:1783–1788
Funding
This study was completed without funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lohrisch, C., Francl, M., Sun, S. et al. Willingness of breast cancer patients to undergo biopsy and breast cancer clinicians’ practices around seeking biopsy at the time of breast cancer relapse. Breast Cancer Res Treat 168, 221–228 (2018). https://doi.org/10.1007/s10549-017-4586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4586-9