Abstract
Purpose
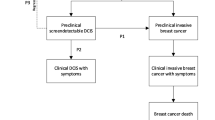
Due to limitations in the ability to identify non-progressive disease, ductal carcinoma in situ (DCIS) is usually managed similarly to localized invasive breast cancer. We used simulation modeling to evaluate the potential impact of a hypothetical test that identifies non-progressive DCIS.
Methods
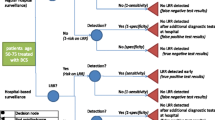
A discrete-event model simulated a cohort of U.S. women undergoing digital screening mammography. All women diagnosed with DCIS underwent the hypothetical DCIS prognostic test. Women with test results indicating progressive DCIS received standard breast cancer treatment and a decrement to quality of life corresponding to the treatment. If the DCIS test indicated non-progressive DCIS, no treatment was received and women continued routine annual surveillance mammography. A range of test performance characteristics and prevalence of non-progressive disease were simulated. Analysis compared discounted quality-adjusted life years (QALYs) and costs for test scenarios to base-case scenarios without the test.
Results
Compared to the base case, a perfect prognostic test resulted in a 40% decrease in treatment costs, from $13,321 to $8005 USD per DCIS case. A perfect test produced 0.04 additional QALYs (16 days) for women diagnosed with DCIS, added to the base case of 5.88 QALYs per DCIS case. The results were sensitive to the performance characteristics of the prognostic test, the proportion of DCIS cases that were non-progressive in the model, and the frequency of mammography screening in the population.
Conclusion
A prognostic test that identifies non-progressive DCIS would substantially reduce treatment costs but result in only modest improvements in quality of life when averaged over all DCIS cases.


Similar content being viewed by others
References
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016) SEER Cancer Statistics Review, 1975–2013
Miglioretti DL, Zhu W, Kerlikowske K, Sprague BL, Onega T, Buist DS, Henderson LM, Smith RA, Breast Cancer Surveillance C (2015) Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol 1:1069–1077. https://doi.org/10.1001/jamaoncol.2015.3084
Sprague BL, McLaughlin V, Hampton JM, Newcomb PA, Trentham-Dietz A (2013) Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast Cancer Res Treat 141:145–154. https://doi.org/10.1007/s10549-013-2670-3
Sprague BL, Trentham-Dietz A (2009) Prevalence of breast carcinoma in situ in the United States. JAMA 302:846–848
Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ (2011) Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat 129:165–173. https://doi.org/10.1007/s10549-011-1430-5
Yen MF, Tabar L, Vitak B, Smith RA, Chen HH, Duffy SW (2003) Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer 39:1746–1754
Independent U. K. Panel on Breast Cancer Screening (2012) The benefits and harms of breast cancer screening: an independent review. Lancet 380:1778–1786. https://doi.org/10.1016/S0140-6736(12)61611-0
Etzioni R, Gulati R, Mallinger L, Mandelblatt J (2013) Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med 158:831–838. https://doi.org/10.7326/0003-4819-158-11-201306040-00008
Shiyanbola OO, Sprague BL, Hampton JM, Dittus K, James TA, Herschorn S, Gangnon RE, Weaver DL, Trentham-Dietz A (2016) Emerging trends in surgical and adjuvant radiation therapies among women diagnosed with ductal carcinoma in situ. Cancer 122:2810–2818. https://doi.org/10.1002/cncr.30105
Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW Jr, Davidson NE, Ingle JN, Perez EA, Wood WC, Sparano JA, Badve S (2013) A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 105:701–710. https://doi.org/10.1093/jnci/djt067
Lagios MD, Silverstein MJ (2014) Risk of recurrence of ductal carcinoma in situ by oncotype Dx technology: some concerns. Cancer 120:1085. https://doi.org/10.1002/cncr.28523
Rakovitch E, Nofech-Mozes S, Hanna W, Baehner FL, Saskin R, Butler SM, Tuck A, Sengupta S, Elavathil L, Jani PA, Bonin M, Chang MC, Robertson SJ, Slodkowska E, Fong C, Anderson JM, Jamshidian F, Miller DP, Cherbavaz DB, Shak S, Paszat L (2015) A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat 152:389–398. https://doi.org/10.1007/s10549-015-3464-6
Alvarado M, Carter DL, Guenther JM, Hagans J, Lei RY, Leonard CE, Manders J, Sing AP, Broder MS, Cherepanov D, Chang E, Eagan M, Hsiao W, Schultz MJ (2015) The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: a prospective clinical utility assessment of the 12-gene DCIS score result. J Surg Oncol 111:935–940. https://doi.org/10.1002/jso.23933
Molinaro AM, Sison JD, Ljung BM, Tlsty TD, Kerlikowske K (2016) Risk prediction for local versus regional/metastatic tumors after initial ductal carcinoma in situ diagnosis treated by lumpectomy. Breast Cancer Res Treat 157:351–361. https://doi.org/10.1007/s10549-016-3814-z
Benson JR, Wishart GC (2013) Predictors of recurrence for ductal carcinoma in situ after breast-conserving surgery. Lancet Oncol 14:e348–e357. https://doi.org/10.1016/S1470-2045(13)70135-9
Yi M, Meric-Bernstam F, Kuerer HM, Mittendorf EA, Bedrosian I, Lucci A, Hwang RF, Crow JR, Luo S, Hunt KK (2012) Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol 30:600–607. https://doi.org/10.1200/JCO.2011.36.4976
Alagoz O, Ergun MA, Cevik M, Sprague BL, Fryback DG, Gangnon RE, Hampton JM, Stout NK, Trentham-Dietz A (in press) The University of Wisconsin breast cancer epidemiology simulation model: an update. Medical decision making
Trentham-Dietz A, Kerlikowske K, Stout NK, Miglioretti DL, Schechter CB, Ergun MA, van den Broek JJ, Alagoz O, Sprague BL, van Ravesteyn NT, Near AM, Gangnon RE, Hampton JM, Chandler Y, de Koning HJ, Mandelblatt JS, Tosteson AN, Breast Cancer Surveillance Consortium, the Cancer Intervention Surveillance Modeling Network (2016) Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med 165:700–712. https://doi.org/10.7326/M16-0476
Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, Buist DS, Cevik M, Chisholm G, de Koning HJ, Huang H, Hubbard RA, Miglioretti DL, Munsell MF, Trentham-Dietz A, van Ravesteyn NT, Tosteson AN, Mandelblatt JS (2014) Benefits, harms, and costs for breast cancer screening after U.S. implementation of digital mammography. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju092
Lee CI, Cevik M, Alagoz O, Sprague BL, Tosteson AN, Miglioretti DL, Kerlikowske K, Stout NK, Jarvik JG, Ramsey SD, Lehman CD (2015) Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 274:772–780. https://doi.org/10.1148/radiol.14141237
Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, Lee CI, van den Broek JJ, Miglioretti DL, Mandelblatt JS, de Koning HJ, Kerlikowske K, Lehman CD, Tosteson AN (2015) Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 162:157–166. https://doi.org/10.7326/M14-0692
Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, Trentham-Dietz A, Munoz D, Lee SJ, Berry DA, van Ravesteyn NT, Alagoz O, Kerlikowske K, Tosteson AN, Near AM, Hoeffken A, Chang Y, Heijnsdijk EA, Chisholm G, Huang X, Huang H, Ergun MA, Gangnon R, Sprague BL, Plevritis S, Feuer E, de Koning HJ, Cronin KA (2016) collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med 164:215–225. https://doi.org/10.7326/M15-1536
Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, Berry DA, Burnside ES, Chang Y, Chisholm G, de Koning HJ, Ali Ergun M, Heijnsdijk EA, Huang H, Stout NK, Sprague BL, Trentham-Dietz A, Mandelblatt JS, Plevritis SK (2014) Effects of screening and systemic adjuvant therapy on ER-specific U.S. breast cancer mortality. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju289
Batina NG, Trentham-Dietz A, Gangnon RE, Sprague BL, Rosenberg MA, Stout NK, Fryback DG, Alagoz O (2013) Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat 138:519–528. https://doi.org/10.1007/s10549-013-2435-z
Mandelblatt J, Cronin KA, De Koning H, Miglioretti DL, Schechter C, Stout N, Breast Working Group of the Cancer Intevention and Surveillance Modeling Network (CISNET) and the Breast Cancer Surveillance Consortium (BCSC) (AHRQ Publication No. 14-05201-EF-4, December 2015. Collaborative Modeling of U.S. Breast Cancer Screening Strategies. In: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services, Rockville, MD. http://www.uspreventiveservicestaskforce.org/Home/GetFile/1/16255/collabmodelingbc/pdf. Accessed May 2016
Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, Trentham-Dietz A (2015) The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomark Prev 24:905–912. https://doi.org/10.1158/1055-9965.EPI-14-1286
Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL (2006) The Wisconsin breast cancer epidemiology simulation model. J Natl Cancer Inst Monogr 36:37–47
Shwartz M (1978) A mathematical model used to analyze breast cancer screening strategies. Oper Res 26:937–955
Shwartz M (1981) Validation and use of a mathematical model to estimate the benefits of screening younger women for breast cancer. Cancer Detect Prev 4:595–601
Cronin KA, Mariotto AB, Clarke LD, Feuer EJ (2006) Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr 36:26–29
Cronin KA, Yu B, Krapcho M, Miglioretti DL, Fay MP, Izmirlian G, Ballard-Barbash R, Geller BM, Feuer EJ (2005) Modeling the dissemination of mammography in the United States. Cancer Causes Control 16:701–712
Early Breast Cancer Trialists’ Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. https://doi.org/10.1016/S0140-6736(11)61625-5
Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG (2006) Report of nationally representative values for the noninstitutionalized U.S. adult population for 7 health-related quality-of-life scores. Med Decis Making 26:391–400. https://doi.org/10.1177/0272989X06290497
Mariotto AB, Feuer EJ, Harlan LC, Abrams J (2006) Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975-1999. J Natl Cancer Inst Monogr 36:7–15
Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG (2006) Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst 98:774–782
Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML (2008) Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100:630–641. https://doi.org/10.1093/jnci/djn103
Mandelblatt J, Near A, Miglioretti DL, Munoz D, Sprague B, Trentham Dietz A, Gangnon R, Kurian A, Weedon-Fekjaer H, Cronin K, Plevritis SK (in press) Common model inputs in collaborative breast cancer modeling. Med Decis Making
Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham Dietz A (in press) Contribution of breast cancer to overall mortality for U. S. women. Med Decis Making
Munoz D, Plevritis S (in press) Estimating breast cancer progression features and survival by molecular subtype in the absence of screening and treatment. Med Decis Making
Cronin KA, Feuer EJ, Clarke LD, Plevritis SK (2006) Impact of adjuvant therapy and mammography on U.S. mortality from 1975 to 2000: comparison of mortality results from the CISNET breast cancer base case analysis. J Natl Cancer Inst Monogr 36:112–121
National Comprehensive Cancer Network (2015) NCCN clinical practice guidelines in oncology: breast cancer
Rosenberg MA (2006) The impact of mammography and adjuvant therapy on U.S. breast cancer mortality (1975–2000): collective results from the cancer intervention and surveillance modeling network. Competing risks to breast cancer mortality. J Natl Cancer Inst Monogr 36:15–19
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
de Koning HJ, van Ineveld BM, van Oortmarssen GJ, de Haes JC, Collette HJ, Hendriks JH, van der Maas PJ (1991) Breast cancer screening and cost-effectiveness; policy alternatives, quality of life considerations and the possible impact of uncertain factors. Int J Cancer 49:531–537
Grann VR, Patel PR, Jacobson JS, Warner E, Heitjan DF, Ashby-Thompson M, Hershman DL, Neugut AI (2011) Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 125:837–847. https://doi.org/10.1007/s10549-010-1043-4
Roberts A, Habibi M, Frick KD (2014) Cost-effectiveness of contralateral prophylactic mastectomy for prevention of contralateral breast cancer. Ann Surg Oncol 21:2209–2217. https://doi.org/10.1245/s10434-014-3588-7
Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM (2007) Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat 105:195–207
Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K (2010) Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 152:238–246. https://doi.org/10.7326/0003-4819-152-1-201001050-00190
Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C, Roberts T, Pirrie S, Gaunt C, Young J, Billingham L, Dodwell D, Hanby A, Pinder SE, Evans A, Reed M, Jenkins V, Matthews L, Wilcox M, Fairbrother P, Bowden S, Rea D (2015) Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 51:2296–2303. https://doi.org/10.1016/j.ejca.2015.07.017
Acknowledgements
We thank Berta Geller, Julie McGregor, Kathy Peck, Oyewale Shiyanbola, Dawn Pelkey, and Kathleen Howe for their advice, project management, and assistance with data for this project.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health for the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) Program (Grant No. U54 CA163303) and other projects (Grant Nos. U01 CA199218, P30 CA014520, and U54 CA163307). Data collection for model inputs from the Breast Cancer Surveillance Consortium (BCSC) was supported by the National Cancer Institute Grant P01 CA154292, contract HSN261201100031C, and Grant U54 CA163303. The collection of BCSC cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html.
Author information
Authors and Affiliations
Contributions
The authors are solely responsible for the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Corresponding authors
Ethics declarations
Conflicts of interest
Dr. Herschorn reports previously owning stock and currently serving as an unpaid advisor for Hologic. All other authors have no conflicts to disclose.
Ethical approval
The University of Wisconsin Health Sciences Human Subjects Committee determined that this study was exempt from review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trentham-Dietz, A., Ergun, M.A., Alagoz, O. et al. Comparative effectiveness of incorporating a hypothetical DCIS prognostic marker into breast cancer screening. Breast Cancer Res Treat 168, 229–239 (2018). https://doi.org/10.1007/s10549-017-4582-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4582-0