Abstract
Purpose
The BRCA1-like profile identifies tumors with a defect in homologous recombination due to inactivation of BRCA1. This profile has been shown to predict which stage III breast cancer patients benefit from myeloablative, DNA double-strand-break-inducing chemotherapy. We tested the predictive potential of the BRCA1-like profile for adjuvant non-myeloablative, intensified dose-dense chemotherapy in the GAIN trial.
Methods
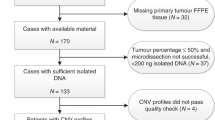
Lymph node positive breast cancer patients were randomized to 3 × 3 dose-dense cycles of intensified epirubicin, paclitaxel, and cyclophosphamide (ETC) or 4 cycles concurrent epirubicin and cyclophosphamide followed by 10 cycles of weekly paclitaxel combined with 4 cycles capecitabine (EC-TX). Only triple negative breast cancer patients (TNBC) for whom tissue was available were included in these planned analyses. BRCA1-like or non-BRCA1-like copy number profiles were derived from low coverage sequencing data.
Results
119 out of 163 TNBC patients (73%) had a BRCA1-like profile. After median follow-up of 83 months, disease free survival (DFS) was not significantly different between BRCA1-like and non-BRCA1-like patients [adjusted hazard ratio (adj.HR) 1.02; 95% confidence interval (CI) 0.55–1.86], neither was overall survival (OS; adj.HR 1.26; 95% CI 0.58–2.71). When split by BRCA1-like status, DFS and OS were not significantly different between treatments. However, EC-TX seemed to result in a trend to an improvement in DFS in patients with a BRCA1-like tumor, while the reverse accounted for ETC treatment in patients with a non-BRCA1-like tumor (p for interaction = 0.094).
Conclusions
The BRCA1-like profile is not associated with survival benefit for a non-myeloablative, intensified regimen in this study population. Considering the limited cohort size, capecitabine might have additional benefit for TNBC patients.



Similar content being viewed by others
References
Brohet RM, Velthuizen ME, Hogervorst FB, Meijers-Heijboer HE, Seynaeve C, Collee MJ, Verhoef S, Ausems MG, Hoogerbrugge N, van Asperen CJ, Gomez Garcia E, Menko F, Oosterwijk JC et al (2014) Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet 51:98–107. doi:10.1136/jmedgenet-2013-101974
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, Evans DG, Izatt L, Eeles RA, Adlard J, Davidson R, Eccles D, Cole T et al (2013) Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 105:812–822. doi:10.1093/jnci/djt095
Vos JR, Hsu L, Brohet RM, Mourits MJ, de Vries J, Malone KE, Oosterwijk JC, de Bock GH (2015) Bias correction methods explain much of the variation seen in breast cancer risks of BRCA1/2 mutation carriers. J Clin Oncol 33:2553–2562. doi:10.1200/JCO.2014.59.0463
Lord CJ, Ashworth A (2016) BRCAness revisited. Nat Rev Cancer 16:110–120. doi:10.1038/nrc.2015.21
Turner N, Tutt A, Ashworth A (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4:814–819. doi:10.1038/nrc1457
Joosse SA, van Beers EH, Tielen IH, Horlings H, Peterse JL, Hoogerbrugge N, Ligtenberg MJ, Wessels LF, Axwijk P, Verhoef S, Hogervorst FB, Nederlof PM (2009) Prediction of BRCA1-association in hereditary non-BRCA1/2 breast carcinomas with array-CGH. Breast Cancer Res Treat 116:479–489. doi:10.1007/s10549-008-0117-z
Wessels LF, van Welsem T, Hart AA, van’t Veer LJ, Reinders MJ, Nederlof PM (2002) Molecular classification of breast carcinomas by comparative genomic hybridization: a specific somatic genetic profile for BRCA1 tumors. Cancer Res 62:7110–7117
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, Van Loo P, Ju YS, Smid M et al (2016) Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534:47–54. doi:10.1038/nature17676
Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, Imholz AL, Wesseling J, Rodenhuis S, Nederlof PM (2013) Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer 108:2172–2177. doi:10.1038/bjc.2013.144
Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, Cornelissen S, Holtkamp M, Froklage FE, de Vries EG, Schrama JG, Wesseling J, van de Vijver MJ et al (2011) An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol 22:1561–1570. doi:10.1093/annonc/mdq624
Schouten PC, Marme F, Aulmann S, Sinn HP, van Essen HF, Ylstra B, Hauptmann M, Schneeweiss A, Linn SC (2015) Breast cancers with a BRCA1-like DNA copy number profile recur less often than expected after high-dose alkylating chemotherapy. Clin Cancer Res 21:763–770. doi:10.1158/1078-0432.CCR-14-1894
Schouten PC, Gluz O, Harbeck N, Mohrmann S, Diallo-Danebrock R, Pelz E, Kruizinga J, Velds A, Nieuwland M, Kerkhoven RM, Liedtke C, Frick M, Kates R et al (2016) BRCA1-like profile predicts benefit of tandem high dose epirubicin-cyclophospamide-thiotepa in high risk breast cancer patients randomized in the WSG-AM01 trial. Int J Cancer 139:882–889. doi:10.1002/ijc.30078
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. doi:10.1038/nrclinonc.2016.66
Bramati A, Girelli S, Torri V, Farina G, Galfrascoli E, Piva S, Moretti A, Dazzani MC, Sburlati P, La Verde NM (2014) Efficacy of biological agents in metastatic triple-negative breast cancer. Cancer Treat Rev 40:605–613. doi:10.1016/j.ctrv.2014.01.003
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. doi:10.1016/S0140-6736(11)61625-5
Guan X, Ma F, Fan Y, Zhu W, Hong R, Xu B (2015) Platinum-based chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis of randomized-controlled trials. Anticancer Drugs 26:894–901. doi:10.1097/CAD.0000000000000260
Hudis CA, Gianni L (2011) Triple-negative breast cancer: an unmet medical need. Oncologist 16(Suppl 1):1–11. doi:10.1634/theoncologist.2011-S1-01
Joensuu H, Gligorov J (2012) Adjuvant treatments for triple-negative breast cancers. Ann Oncol 23(Suppl 6):vi40-5. doi:10.1093/annonc/mds194
Li Q, Jiang Y, Wei W, Yang H, Liu J (2013) Clinical efficacy of including capecitabine in neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 8:e53403. doi:10.1371/journal.pone.0053403
Wang Y, Yang H, Wei JF, Meng L (2012) Efficacy and toxicity of capecitabine-based chemotherapy in patients with metastatic or advanced breast cancer: results from ten randomized trials. Curr Med Res Opin 28:1911–1919. doi:10.1185/03007995.2012.748655
Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, Ahlgren J, Auvinen P, Paija O, Helle L, Villman K, Nyandoto P, Nilsson G et al (2012) Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: final analysis of the randomized FinXX trial. J Clin Oncol 30:11–18. doi:10.1200/JCO.2011.35.4639
Toi M, Lee S-J, Lee ES, Ohtani S, Im Y-H, Im S-A, Park B-W, Kim S-B, Yanagita Y, Takao S, Ohno S, Aogi K, Iwata H, Kim A, Sasano H, Yokota I, Ohashi Y, Masuda N (2016) A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive disease after neoadjuvant chemotherapy (CREATE-X, JBCRG-04). Cancer Res 76:S1–S07
von Minckwitz G, Mobus V, Schneeweiss A, Huober J, Thomssen C, Untch M, Jackisch C, Diel IJ, Elling D, Conrad B, Kreienberg R, Muller V, Luck HJ et al (2013) German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol 31:3531–3539. doi:10.1200/JCO.2012.47.2167
Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, Kuhn W, Nitz U, Schneeweiss A, Huober J, Harbeck N, von Minckwitz G, Runnebaum IB et al (2010) Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 28:2874–2880. doi:10.1200/JCO.2009.24.7643
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Statistics Subcommittee of the NCIEWGoCD. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067–9072. doi:10.1200/JCO.2004.01.0454
Beelen K, Opdam M, Severson TM, Koornstra RH, Vincent AD, Hauptmann M, van Schaik RH, Berns EM, Vermorken JB, van Diest PJ, Linn SC (2013) CYP2C19 2 predicts substantial tamoxifen benefit in postmenopausal breast cancer patients randomized between adjuvant tamoxifen and no systemic treatment. Breast Cancer Res Treat 139:649–655. doi:10.1007/s10549-013-2568-0
Schouten PC, Grigoriadis A, Kuilman T, Mirza H, Watkins JA, Cooke SA, van Dyk E, Severson TM, Rueda OM, Hoogstraat M, Verhagen CV, Natrajan R, Chin SF et al (2015) Robust BRCA1-like classification of copy number profiles of samples repeated across different datasets and platforms. Mol Oncol 9:1274–1286. doi:10.1016/j.molonc.2015.03.002
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi:10.1093/bioinformatics/btp324
Benjamini Y, Speed TP (2012) Summarizing and correcting the GC content bias in high-throughput sequencing. Nucleic Acids Res 40:e72. doi:10.1093/nar/gks001
Hryniuk W, Frei E 3rd, Wright FA (1998) A single scale for comparing dose-intensity of all chemotherapy regimens in breast cancer: summation dose-intensity. J Clin Oncol 16:3137–3147
Akashi-Tanaka S, Watanabe C, Takamaru T, Kuwayama T, Ikeda M, Ohyama H, Mori M, Yoshida R, Hashimoto R, Terumasa S, Enokido K, Hirota Y, Okuyama H et al (2015) BRCAness predicts resistance to taxane-containing regimens in triple negative breast cancer during neoadjuvant chemotherapy. Clin Breast Cancer 15:80–85. doi:10.1016/j.clbc.2014.08.003
Fisher B, Anderson S, Wickerham DL, DeCillis A, Dimitrov N, Mamounas E, Wolmark N, Pugh R, Atkins JN, Meyers FJ, Abramson N, Wolter J, Bornstein RS et al (1997) Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol 15:1858–1869
Fisher B, Anderson S, DeCillis A, Dimitrov N, Atkins JN, Fehrenbacher L, Henry PH, Romond EH, Lanier KS, Davila E, Kardinal CG, Laufman L, Pierce HI et al (1999) Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol 17:3374–3388
Joensuu H, Kellokumpu-Lehtinen P-L, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, Auvinen P, Lahdenperä O, Villman K, Nyandoto P, Nilsson G, Murashev M, Poikonen-Saksela P, Bono P, Kataja V, Lindman H (2016) Adjuvant capesitabine in combination with docetaxel (T), epirubicin (E), and cyclophosphamide (C) in the treatment of early breast cancer (BC): 10-year survival results from the randomized FinXX trial. J Clin Oncol 34:1001
O’Shaughnessy J, Koeppen H, Xiao Y, Lackner MR, Paul D, Stokoe C, Pippen J Jr, Krekow L, Holmes FA, Vukelja S, Lindquist D, Sedlacek S, Rivera R et al (2015) Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clin Cancer Res 21:4305–4311. doi:10.1158/1078-0432.CCR-15-0636
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338. doi:10.1038/nrc1074
Smorenburg CH, Sparreboom A, Bontenbal M, Verweij J (2001) Combination chemotherapy of the taxanes and antimetabolites: its use and limitations. Eur J Cancer 37:2310–2323
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Narod SA, Lubinski J, Polish Hereditary Breast Cancer C (2008) Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat 108:289–296. doi:10.1007/s10549-007-9600-1
Kriege M, Jager A, Hooning MJ, Huijskens E, Blom J, van Deurzen CH, Bontenbal M, Collee JM, Menke-Pluijmers MB, Martens JW, Seynaeve C (2012) The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer 118:899–907. doi:10.1002/cncr.26351
Simon RM, Paik S, Hayes DF (2009) Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101:1446–1452. doi:10.1093/jnci/djp335
Acknowledgments
Our gratitude goes to all patients who participated in the GAIN trial. Also, we would like to thank the Genomics Core Facility of the Netherlands Cancer Institute for generating the sequencing data.
Funding
This study was funded by Zaan de Wandel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All remaining authors have declared no conflicts of interest.
Disclosures
SCL is named inventor on patent applications for the BRCA1-like signature (WO/2015/080585 and PCT/NL2014/050813). SCL is an advisory board member for Cergentis, Novartis, Roche, and Sanofi. SCL received research support funding from Amgen, AstraZeneca, Genentech, Roche, and Sanofi. VJM is an advisory board member for Myelo Therapeutics GmbH and Amgen. CS received honoraria from Roche and AstraZeneca. CT received honoraria from Amgen and TEVA. CK received honoraria from Merck, Servier, Roche and Pfizer and was paid to participate in a speaker’s bureau for Merck and Amgen.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Rossum, A.G.J., Schouten, P.C., Weber, K.E. et al. BRCA1-like profile is not significantly associated with survival benefit of non-myeloablative intensified chemotherapy in the GAIN randomized controlled trial. Breast Cancer Res Treat 166, 775–785 (2017). https://doi.org/10.1007/s10549-017-4444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4444-9