Abstract
Purpose
Ki67 is a proliferation marker commonly assessed by immunohistochemistry in breast cancer, and it has been proposed as a clinical marker for subtype classification, prognosis, and prediction of therapeutic response. However, the clinical utility of Ki67 is limited by the lack of consensus on the optimal cut point for each application.
Methods
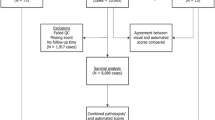
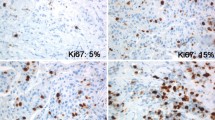
We assessed Ki67 by immunohistochemistry using Definiens digital image analysis (DIA) in 2653 cases of incident invasive breast cancer diagnosed in the Nurses’ Health Study from 1976 to 2006. Ki67 was scored as continuous percentage of positive tumor cells, and dichotomized at various cut points. Multivariable hazard ratios (HR) and 95% confidence intervals (CI) were calculated using Cox regression models for distant recurrence, breast cancer-specific mortality and overall mortality in relation to luminal subtypes defined with various Ki67 cut points, adjusting for breast cancer prognostic factors, clinico-pathologic features and treatment.
Results
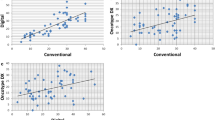
DIA was highly correlated with manual scoring of Ki67 (Spearman correlation ρ = 0.86). Mean Ki67 score was higher in grade-defined luminal B (12.6%), HER2-enriched (17.9%) and basal-like (20.6%) subtypes compared to luminal A (8.9%). In multivariable-adjusted models, luminal B tumors had higher breast cancer-specific mortality compared to luminal A cancer classified using various cut points for Ki67 positivity including the 14% cut point routinely reported in the literature (HR 1.38, 95% CI 1.11–1.72, p = 0.004). There was no significant difference in clinical outcomes for ER− tumors according to Ki67 positivity defined at various cut points.
Conclusions
Assessment of Ki67 in breast tumors by DIA was a robust and quantitative method. Results from this large prospective cohort study provide support for the clinical relevance of using Ki67 at the 14% cut point for luminal subtype classification and breast cancer prognosis.

Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CK5/6:
-
Cytokeratin 5/6
- DAB:
-
Diaminobenzidine
- DIA:
-
Digital image analysis
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Estrogen receptor
- FFPE:
-
Formalin-fixed paraffin-embedded
- HER2:
-
Human epidermal growth factor receptor 2
- NHS:
-
Nurses’ Health Study
- IHC:
-
Immunohistochemistry
- HR:
-
Hazard ratio
- PMH:
-
Post-menopausal hormone
- PR:
-
Progesterone receptor
- TMA:
-
Tissue microarray
- TNBC:
-
Triple-negative breast cancer
References
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A et al (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100(18):10393–10398
Kao KJ, Chang KM, Hsu HC, Huang AT (2011) Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer 11(143):1471–2407
Prat A, Lluch A, Albanell J, Barry WT, Fan C, Chacon JI et al (2014) Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br J Cancer 111(8):1532–1541
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN (2009) Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 7(1–2):4–13
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M et al (2015) Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26(8):1533–1546
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295(21):2492–2502
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Simpson JF, Gray R, Dressler LG, Cobau CD, Falkson CI, Gilchrist KW et al (2000) Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol 18(10):2059–2069
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Schonk DM, Kuijpers HJ, van Drunen E, van Dalen CH, Geurts van Kessel AH, Verheijen R et al (1989) Assignment of the gene(s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet 83(3):297–299
Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T (2006) Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol 206(3):624–635
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23(28):7212–7220
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906
Zhang X, Giovannucci EL, Wu K, Smith-Warner SA, Fuchs CS, Pollak M et al (2012) Magnesium intake, plasma C-peptide, and colorectal cancer incidence in US women: a 28-year follow-up study. Br J Cancer 106(7):1335–1341
Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y et al (2008) Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 10(4):R67
Healey MA, Hu R, Beck AH, Collins LC, Schnitt SJ, Tamimi RM et al (2014) Association of H3K9me3 and H3K27me3 repressive histone marks with breast cancer subtypes in the Nurses’ Health Study. Breast Cancer Res Treat 147(3):639–651
Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM (2011) Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol 24(7):924–931
Zabaglo L, Salter J, Anderson H, Quinn E, Hills M, Detre S et al (2010) Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol 63(9):800–804
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479–2486
Colditz GA, Rosner B (2000) Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol 152(10):950–964
Kroenke CH, Chen WY, Rosner B, Holmes MD (2005) Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370–1378
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691
Engstrom MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA et al (2013) Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat 140(3):463–473
Parise CA, Caggiano V (2014) Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol 2014:469251
Yanagawa M, Ikemot K, Kawauchi S, Furuya T, Yamamoto S, Oka M et al (2012) Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res Notes 5:376
Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL (2011) Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol 19(5):431–436
Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH et al (2011) Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 13(2):R22
Huang L, Liu Z, Chen S, Liu Y, Shao Z (2013) A prognostic model for triple-negative breast cancer patients based on node status, cathepsin-D and Ki-67 index. PLoS One 8(12):e83081
Abubakar M, Orr N, Daley F, Coulson P, Ali HR, Blows F et al (2016) Prognostic value of automated KI67 scoring in breast cancer: a centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res 18(1):104
Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F et al (2016) Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat 157(2):363–371
Huh SJ, Oh H, Peterson MA, Almendro V, Hu R, Bowden M et al (2016) The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women. Cancer Res 76(7):1926–1934
Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S et al (2014) Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res 16(3):R65
Acknowledgements
We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. This study was supported by the National Cancer Institute (UM1 CA186107 and Dietary and Hormonal Determinants of Cancer in Women NIH P01 CA87969). MAH and KAH were supported by the National Institutes of Health Cancer Epidemiology Training Grant (NIH T32 CA09001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. AHB has an equity interest in PathAI,Inc.
Ethical standards
All data collection was conducted with approval of appropriate institutional review boards to protect human subjects with consent and data protection systems in place. Data analysis for this manuscript was conducted on de-identified data sets.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Healey, M.A., Hirko, K.A., Beck, A.H. et al. Assessment of Ki67 expression for breast cancer subtype classification and prognosis in the Nurses’ Health Study. Breast Cancer Res Treat 166, 613–622 (2017). https://doi.org/10.1007/s10549-017-4421-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4421-3