Abstract
Purpose
The aromatase inhibitors (AI) exemestane (EXE), letrozole (LET), and anastrozole suppress estrogen biosynthesis, and are effective treatments for estrogen receptor (ER)-positive breast cancer. Prior work suggests that anastrozole blood concentrations are associated with the magnitude of estrogen suppression. The objective of this study was to determine whether the magnitude of estrogen suppression, as determined by plasma estradiol (E2) concentrations, in EXE or LET treated patients is associated with plasma AI concentrations.
Methods
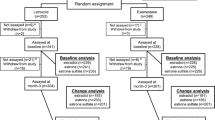
Five hundred post-menopausal women with ER-positive breast cancer were enrolled in the prospective Exemestane and Letrozole Pharmacogenetic (ELPh) Study conducted by the COnsortium on BReast cancer phArmacogomics (COBRA) and randomly assigned to either drug. Estrogen concentrations were measured at baseline and after 3 months of AI treatment and drug concentrations were measured after 1 or 3 months. EXE or LET concentrations were compared with 3-month E2 concentration or the change from baseline to 3 months using several complementary statistical procedures.
Results
Four-hundred patients with on-treatment E2 and AI concentrations were evaluable (EXE n = 200, LET n = 200). Thirty (7.6%) patients (EXE n = 13, LET n = 17) had 3-month E2 concentrations above the lower limit of quantification (LLOQ) (median: 4.75; range: 1.42–63.8 pg/mL). EXE and LET concentrations were not associated with on-treatment E2 concentrations or changes in E2 concentrations from baseline (all p > 0.05).
Conclusions
Steady-state plasma AI concentrations do not explain variability in E2 suppression in post-menopausal women receiving EXE or LET therapy, in contrast with prior evidence in anastrozole treated patients.




Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative G, Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thurlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386(10001):1341–1352. doi:10.1016/S0140-6736(15)61074-1
Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, Colleoni M, Lang I, Gomez HL, Tondini C, Pinotti G, Price KN, Coates AS, Goldhirsch A, Gelber RD (2016) Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. J Clin Oncol 34(19):2221–2231. doi:10.1200/jco.2015.64.3171
Lintermans A, Neven P (2015) Safety of aromatase inhibitor therapy in breast cancer. Expert Opin Drug Saf 14(8):1201–1211. doi:10.1517/14740338.2015.1053458
Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE (1996) Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer 74(8):1286–1291
Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20(3):751–757
Henry NL, Giles JT, Stearns V (2008) Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology 22(12):1401–1408
Lintermans A, Neven P (2011) Pharmacology of arthralgia with estrogen deprivation. Steroids 76(8):781–785. doi:10.1016/j.steroids.2011.02.034
Robarge JD, Desta Z, Nguyen AT, Li L, Hertz D, Rae JM, Hayes DF, Storniolo AM, Stearns V, Flockhart DA, Skaar TC, Henry NL (2017) Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer. Breast Cancer Res Treat 161(3):453–461. doi:10.1007/s10549-016-4077-4
Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, Barman P, Dudenkov TT, Northfelt DW, Perez EA, Flockhart DA, Williard CV, Wang L, Weinshilboum RM (2015) Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids 99:32–38. doi:10.1016/j.steroids.2014.08.007
Henry NL, Giles J, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes D, Storniolo AM, Clauw D (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111(2):365–372. doi:10.1007/s10549-007-9774-6
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942. doi:10.1200/JCO.2011.38.0261
Henry NL, Chan HP, Dantzer J, Goswami CP, Li L, Skaar TC, Rae JM, Desta Z, Khouri N, Pinsky R, Oesterreich S, Zhou C, Hadjiiski L, Philips S, Robarge J, Nguyen AT, Storniolo AM, Flockhart DA, Hayes DF, Helvie MA, Stearns V (2013) Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. Br J Cancer 109(9):2331–2339. doi:10.1038/bjc.2013.587
Henry NL, Skaar TC, Dantzer J, Li L, Kidwell K, Gersch C, Nguyen AT, Rae JM, Desta Z, Oesterreich S, Philips S, Carpenter JS, Storniolo AM, Stearns V, Hayes DF, Flockhart DA (2013) Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res Treat 138(3):807–816. doi:10.1007/s10549-013-2504-3
Oesterreich S, Henry NL, Kidwell KM, Van Poznak CH, Skaar TC, Dantzer J, Li L, Hangartner TN, Peacock M, Nguyen AT, Rae JM, Desta Z, Philips S, Storniolo AM, Stearns V, Hayes DF, Flockhart DA (2015) Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res Treat 154(2):263–273. doi:10.1007/s10549-015-3608-8
Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72(8):666–671. doi:10.1016/j.steroids.2007.05.003
Hertz DL, Kidwell KM, Seewald NJ, Gersch CL, Desta Z, Flockhart DA, Storniolo AM, Stearns V, Skaar TC, Hayes DF, Henry NL, Rae JM (2016) Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J. doi:10.1038/tpj.2016.60
Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA (2011) Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 90(5):693–700. doi:10.1038/clpt.2011.174
Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M (1998) In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res 4(9):2089–2093
Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, Northfelt DW, Olson JE, Perez EA, Desta Z, Weintraub RA, Williard CV, Flockhart DA, Weinshilboum RM (2010) Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Can Res 70(8):3278–3286. doi:10.1158/0008-5472.CAN-09-3024
Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, Aas T, Lonning PE (2008) Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res 14(19):6330–6335. doi:10.1158/1078-0432.ccr-07-5221
McCormack JP, Jewesson PJ (1992) A critical reevaluation of the “therapeutic range” of aminoglycosides. Clin Infect Dis 14(1):320–339
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478. doi:10.1007/s10549-012-2114-5
Smith IE, Dowsett M, Yap YS, Walsh G, Lonning PE, Santen RJ, Hayes D (2006) Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 24(16):2444–2447. doi:10.1200/jco.2005.05.3694
Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, Desta Z, Rae JM, Otte JL, Carpenter JS, Storniolo AM, Hayes DF, Stearns V, Henry NL (2016) Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist 21(5):539–546. doi:10.1634/theoncologist.2015-0349
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. doi:10.1016/s0140-6736(11)60993-8
Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R, D’Souza DP, Chalchal H, Spadafora S, Stearns V, Perez EA, Liedke PE, Lang I, Elliott C, Gelmon KA, Chapman JA, Shepherd LE (2013) Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27–a randomized controlled phase III trial. J Clin Oncol 31(11):1398–1404. doi:10.1200/jco.2012.44.7805
O’Shaughnessy J, Yardley D, Burris H, De Boer R, Amadori D, McIntyre K, Ejlertsen B, Gnant M, Jonat W, Pritchard K, Dowsett M, Hart L, Poggio S, Valagussa P, Salomon H, Wamil B, Smith I (2016) Abstract PD2-01: Randomized phase 3 trial of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor positive, node positive early breast cancer: Final efficacy and safety results of the femara versus anastrozole clinical evaluation (Face) trial. Cancer Research 76 (4 Supplement):PD2-01-PD02-01. doi:10.1158/1538-7445.sabcs15-pd2-01
Smith I, Yardley D, Burris H, De Boer R, Amadori D, McIntyre K, Ejlertsen B, Gnant M, Jonat W, Pritchard KI, Dowsett M, Hart L, Poggio S, Comarella L, Salomon H, Wamil B, O’Shaughnessy J (2017) Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. doi:10.1200/jco.2016.69.2871
Wang J, Lu K, Song Y, Zhao S, Ma W, Xuan Q, Tang D, Zhao H, Liu L, Zhang Q (2015) RANKL and OPG polymorphisms are associated with aromatase inhibitor-related musculoskeletal adverse events in chinese han breast cancer patients. PLoS ONE 10(7):e0133964. doi:10.1371/journal.pone.0133964
Lintermans A, Vanderschueren D, Verhaeghe J, Van Asten K, Jans I, Van Herck E, Laenen A, Paridaens R, Billen J, Pauwels S, Vermeersch P, Wildiers H, Christiaens MR, Neven P (2014) Arthralgia induced by endocrine treatment for breast cancer: a prospective study of serum levels of insulin like growth factor-I, its binding protein and oestrogens. Eur J Cancer 50(17):2925–2931. doi:10.1016/j.ejca.2014.08.012
Henry NL, Conlon A, Kidwell KM, Griffith K, Smerage JB, Schott AF, Hayes DF, Williams DA, Clauw DJ, Harte SE (2014) Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J Pain 15(5):468–475. doi:10.1016/j.jpain.2014.01.487
Wang JZ, Deogan MS, Lewis JR, Chew S, Mullin BH, McNab TJ, Wilson SG, Ingley E, Prince RL (2011) A non-synonymous coding change in the CYP19A1 gene Arg264Cys (rs700519) does not affect circulating estradiol, bone structure or fracture. BMC Med Genet. doi:10.1186/1471-2350-12-165
Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei AA, Weinshilboum RM (2005) Human aromatase: gene resequencing and functional genomics. Can Res 65(23):11071–11082
Hertz DL, Henry NL, Rae JM (2017) Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics 18(5):481–499. doi:10.2217/pgs-2016-0205
Acknowledgements
This research was supported by Pharmacogenetics Research Network Grant No. U-01 GM61373 (D.A.F.) and Clinical Pharmacology Training Grant No. 5T32-GM08425 (D.A.F.) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), from Grants No. M01-RR000042 (University of Michigan), M01-RR00750 (Indiana University), and M01-RR00052 (Johns Hopkins University) from the National Center for Research Resources (NCRR), a component of the NIH, the Breast Cancer Research Foundation (BCRF) (N003173 to JMR and DFH), the National Cancer Institute (5T32CA083654-12, PI Jeremy Taylor), the National Institute of General Medical Sciences (GM099143 to J.M.R.) and the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (P30 CA046592) by the use of the following Cancer Center Core: University of Michigan DNA Sequencing Core. In addition, these studies were supported by grants from Pfizer (D.F.H.), Novartis Pharma AG (D.F.H.), the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (D.F.H.). Drugs were supplied by Novartis and Pfizer. We also wish to posthumously recognize David Flockhart, MD, PhD, who co-chaired the COnsortium on BReast cancer phArmacogomics (COBRA) and passed during preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
DFH and VS have each received research funding from Pfizer and Novartis Pharma AG, including for this study. The authors declare no other competing interest.
Additional information
This work was submitted in preliminary form to 2017 American Society for Clinical Oncology Annual Meeting.
Rights and permissions
About this article
Cite this article
Hertz, D.L., Speth, K.A., Kidwell, K.M. et al. Variable aromatase inhibitor plasma concentrations do not correlate with circulating estrogen concentrations in post-menopausal breast cancer patients. Breast Cancer Res Treat 165, 659–668 (2017). https://doi.org/10.1007/s10549-017-4346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4346-x