Abstract
Purpose
Hepatitis C virus (HCV) is one of the major pathogens of chronic viral hepatitis, and approximately 38 million patients are infected with HCV in China. However, little information is available on the effect of HCV infection during chemotherapy for breast cancer and the impact of HCV infection on the toxicity of chemotherapy and targeted therapy.
Methods
We performed a retrospective survey of 835 patients who were diagnosed with breast cancer between January 2010 and December 2015 at our institution. All patients had been screened for HCV infection at the time of breast cancer diagnosis. We retrospectively investigated the toxicities of chemotherapy and the changes in HCV load based on a review of the medical records.
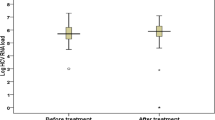
Results
A total of 21 patients with positive anti-HCV antibody tests received chemotherapy. The median patient age was 46.3 ± 11.2 years. Four (19.0%) patients exhibited abnormal liver function at baseline. The morbidity of abnormal liver function at baseline was higher in HCV-infected patients (19.0% vs. 0, P = 0.000). Four patients received trastuzumab therapy. Five (23.8%) patients who received chemotherapy developed hepatitis. No patients presented with HCV reactivation. The morbidity of hepatitis and the rate of disruption of chemotherapy were not significantly different between breast cancer patients without HCV infection and those with HCV infection (23.8 vs. 14.2% P = 0.342, 9.5 vs. 5.0% P = 0.619, respectively).
Conclusion
HCV infection had no adverse impact on chemotherapy in breast cancer patients. However, consulting a gastroenterologist and closely monitoring liver function during the course of chemotherapy may benefit patients.
Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- CHC:
-
Chronic hepatitis C
- HBV:
-
Hepatitis B virus
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER-2:
-
Human epidermal growth factor receptor 2
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- PT:
-
Prothrombin time
References
WHO (2014) Guidelines for the screening, care and treatment of persons with hepatitis C infection. World Health Organization, Geneva
Lalezari J, Box T, O’Riordan W, Mehra P, Nguyen T, Poordad F, Dejesus E, Kwo P, Godofsky E, Lawrence S, Dubuc-Patrick G, Chen J, McCarville J, Pietropaolo K, Zhou XJ, Sullivan-Bólyai J, Mayers D (2013) IDX184 in combination with pegylated interferon-alpha2a and ribavirin for 2 weeks in treatment-naive patients with chronic hepatitis C. Antivir Ther 18:755–764. doi:10.3851/IMP2552
Liu Y, Cai Q, Li X, Shao X, Luo Q, Zhang X, Zhao Z (2014) Effect of drug resistance mutations on antiviral agents in HCV patients. Antivir Ther. doi:10.3851/IMP2852
Zeng H, Zheng R, Zhang S, Zou X, Chen W (2014) Female breast cancer statistics of 2010 in China: estimates based on data from 145 population-based cancer registries. J Thorac Dis 6:466–470. doi:10.3978/j.issn.2072-1439.2014.03.03
Chen W, Zheng R, Zeng H, Zhang S, He J (2015) Annual report on status of cancer in China, 2011. Chin J Cancer Res 27:2–12. doi:10.3978/j.issn.1000-9604.2015.01.06
DeSantis C, Ma J, Bryan L, Jemal A (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64:52–62. doi:10.3322/caac.21203
Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G (2008) Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 148:519–528. doi:10.7326/0003-4819-148-7-200804010-00008
Ennishi D, Maeda Y, Niitsu N, Kojima M, Izutsu K, Takizawa J, Kusumoto S, Okamoto M, Yokoyama M, Takamatsu Y, Sunami K, Miyata A, Murayama K, Sakai A, Matsumoto M, Shinagawa K, Takaki A, Matsuo K, Kinoshita T, Tanimoto M (2010) Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood 116:5119–5125. doi:10.1182/blood-2010-06-289231
Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA (2012) Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol 57:1177–1185. doi:10.1016/j.jhep.2012.07.031
WHO (2015) Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. World Health Organization, Geneva
Miura Y, Theriault RL, Naito Y, Suyama K, Shimomura A, Iwatani T, Miura D, Kawabata H, Kumada H, Takano T (2013) The safety of chemotherapy for breast cancer patients with hepatitis C Virus infection. J Cancer 4:519–523. doi:10.7150/jca.6231
Long M, Jia W, Li S, Jin L, Wu J, Rao N, Feng H, Chen K, Deng H, Liu F, Su F, Song E (2011) A single-center, prospective and randomized controlled study: can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat 127:705–712. doi:10.1007/s10549-011-1455-9
Yeo W, Ho WM, Hui P, Chan PK, Lam KC, Lee JJ, Johnson PJ (2004) Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat 88:209–215. doi:10.1007/s10549-004-0725-1
Wang Y, Luo XM, Yang D, Zhang J, Zhuo HY, Zhang J, Jiang Y (2013) Testing for hepatitis B infection in prospective chemotherapy patients: a retrospective study. World J Gastroenterol 19:923–930. doi:10.3748/wjg.v19.i6.923
Nicot F, Kamar N, Mariamé B, Rostaing L, Pasquier C, Izopet J (2010) No evidence of occult hepatitis C virus (HCV) infection in serum of HCV antibody-positive HCV RNA-negative kidney-transplant patients. Transpl Int 23:594–601. doi:10.1111/j.1432-2277.2009.01025.x
Eisenberg R, Looney RJ (2005) The therapeutic potential of anti-CD20 “what do B-cells do?”. Clin Immunol 117:207–213. doi:10.1016/j.clim.2005.08.006
Baselga J, Perez EA, Pienkowski T, Bell R (2006) Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist 11(Suppl 1):4–12. doi:10.1634/theoncologist.11-90001-4
Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M (2013) Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast 22(Suppl 2):S152–S155. doi:10.1016/j.breast.2013.07.029
Ma B, Yeo W, Hui P, Ho WM, Johnson PJ (2002) Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer: a retrospective review of Chinese patients and comparison with an historic western series. Radiother Oncol 62:185–189. doi:10.1016/S0167-8140(02)00003-8
Kenmotsu H, Tanigawara Y (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the western dose. Cancer Sci 106:497–504. doi:10.1111/cas.12647
Acknowledgements
The conception of the work was performed by Prof. Huang. All authors contributed to data acquisition; each revision was permitted by all authors. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Funding
This study was supported by Guangdong Natural Science Foundation 2014A030313193.
Author information
Authors and Affiliations
Contributions
YL, ZYL, and YH designed the study, YL, ZYL, and XL analyzed the data, and QAH and JNW were in charge of drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Availability of data and material
The datasets collected or analyzed during the current study are available from the corresponding author upon reasonable request.
Yu Liu, Zhan-Yi Li and Jia-Ni Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, ZY., Wang, JN. et al. Effects of hepatitis C virus infection on the safety of chemotherapy for breast cancer patients. Breast Cancer Res Treat 164, 379–383 (2017). https://doi.org/10.1007/s10549-017-4259-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4259-8