Abstract
Purpose
Inter-individual differences in estrogen concentrations during treatment with aromatase inhibitors (AIs) may contribute to therapeutic response and toxicity. The aim of this study was to determine plasma concentrations of estradiol (E2), estrone (E1), and estrone sulfate (E1S) in a large cohort of AI-treated breast cancer patients.
Methods
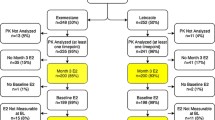
In a randomized, multicenter trial of postmenopausal women with early-stage breast cancer starting treatment with letrozole (n = 241) or exemestane (n = 228), plasma estrogen concentrations at baseline and after 3 months were quantitated using a sensitive mass spectrometry-based assay. Concentrations and suppression below the lower limit of quantification (LLOQ) were compared between estrogens and between drugs.
Results
The ranges of baseline estrogen concentrations were <LLOQ–361 pg/mL for E2, <LLOQ–190 pg/mL for E1, and 8.3–4060 pg/mL for E1S. For E2, the frequency of suppression below the LLOQ was not statistically significantly different between AIs (exemestane: 89.0%, letrozole: 86.9%, p = 0.51). However, patients on letrozole were more likely to achieve suppression below the LLOQ of both E1 (exemestane: 80.1%, letrozole: 90.1%, p = 0.005) and E1S (exemestane: 17.4%, letrozole: 54.9%, p = 4.34e−15). After 3 months of AI therapy, the ranges of estrogen concentrations were <LLOQ–63.8 pg/mL, <LLOQ–36.7 pg/mL, and <LLOQ–1090 pg/mL for E2, E1, and E1S, respectively. During treatment, 16 patients had an increased concentration compared to the baseline concentration of at least one estrogen.
Conclusions
Letrozole had greater suppression of plasma E1 and E1S than exemestane, though the response was highly variable among patients. Additional research is required to examine the clinical relevance of differential estrogen suppression.



Similar content being viewed by others
References
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J et al (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28:3784–3796. doi:10.1200/JCO.2009.26.3756
Early Breast Cancer Trialists’ Collaborative Group, Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC et al (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. doi:10.1016/S0140-6736(15)61074-1
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA et al (2012) Predictors of aromatase inhibitor discontinuation due to treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30:936–942
Felson DT, Cummings SR (2005) Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum 52:2594–2598
Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20:751–757
Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M (1998) In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res 4:2089–2093
Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE (1996) Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer 74:1286–1291
Folkerd EJ, Lonning PE, Dowsett M (2014) Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol 14:1396–1400 [pii] JCO.2013.53.9411
Geisler J, Lonning PE (2005) Endocrine effects of aromatase inhibitors and inactivators in vivo: review of data and method limitations. J Steroid Biochem Mol Biol 95:75–81. doi:10.1016/j.jsbmb.2005.04.015
Stanczyk FZ, Lee JS, Santen RJ (2007) Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomark Prev 16:1713–1719
Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME (2013) Challenges to the measurement of estradiol: an Endocrine Society position statement. J Clin Endocrinol Metab 98:1376–1387. doi:10.1210/jc.2012-3780
Stanczyk FZ, Clarke NJ (2014) Measurement of estradiol–challenges ahead. J Clin Endocrinol Metab 99:56–58. doi:10.1210/jc.2013-2905
Jaque J, Macdonald H, Brueggmann D, Patel SK, Azen C, Clarke N, Stanczyk FZ (2013) Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. Springerplus 2:5. doi:10.1186/2193-1801-2-5
Henry NL, Xia R, Banerjee M, Gersch C, McConnell D, Giacherio D, Schott AF, Pearlman M, Stearns V, Partridge AH et al (2013) Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol 24:2011–2016
Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72:666–671
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372
Henry NL, Chan HP, Dantzer J, Goswami CP, Li L, Skaar TC, Rae JM, Desta Z, Khouri N, Pinsky R et al (2013) Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. Br J Cancer 109:2331–2339. doi:10.1038/bjc.2013.587
Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V et al (2011) Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 90:693–700. doi:10.1038/clpt.2011.174
Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, Northfelt DW, Olson JE, Perez EA, Desta Z et al (2010) Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res 70:3278–3286 [pii] 0008-5472.CAN-09-3024
Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E et al (2006) Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 91:3791–3797
Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, Healey CS, Dunning A, Doody D, Ponder B et al (2006) Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomark Prev 15:1502–1508 [pii] 15/8/1502
Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, Barman P, Dudenkov TT, Northfelt DW, Perez EA et al (2015) Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids 99:32–38. doi:10.1016/j.steroids.2014.08.007
Dixon JM, Renshaw L, Young O, Murray J, Macaskill EJ, McHugh M, Folkerd E, Cameron DA, A’Hern RP, Dowsett M (2008) Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol 26:1671–1676
Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, Aas T, Lonning PE (2008) Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res 14:6330–6335. doi:10.1158/1078-0432.CCR-07-5221
Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R et al (2013) Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27–a randomized controlled phase III trial. J Clin Oncol 31:1398–1404 [pii] JCO.2012.44.7805
Santner SJ, Feil PD, Santen RJ (1984) In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab 59:29–33. doi:10.1210/jcem-59-1-29
Santner SJ, Leszczynski D, Wright C, Manni A, Feil PD, Santen RJ (1986) Estrone sulfate: a potential source of estradiol in human breast cancer tissues. Breast Cancer Res Treat 7:35–44
Lonning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J (2009) Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol 117:31–41. doi:10.1016/j.jsbmb.2009.06.005
James MR, Skaar TC, Lee RY, MacPherson A, Zwiebel JA, Ahluwalia BS, Ampy F, Clarke R (2001) Constitutive expression of the steroid sulfatase gene supports the growth of MCF-7 human breast cancer cells in vitro and in vivo. Endocrinology 142:1497–1505. doi:10.1210/endo.142.4.8091
Wang L, Ellsworth KA, Moon I, Pelleymounter LL, Eckloff BW, Martin YN, Fridley BL, Jenkins GD, Batzler A, Suman VJ et al (2010) Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res 70:319–328. doi:10.1158/0008-5472.CAN-09-3224
Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M (2012) Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol 30:2977–2980. doi:10.1200/JCO.2012.42.0273
Lonning PE, Haynes BP, Dowsett M (2014) Relationship of body mass index with aromatisation and plasma and tissue oestrogen levels in postmenopausal breast cancer patients treated with aromatase inhibitors. Eur J Cancer 50:1055–1064. doi:10.1016/j.ejca.2014.01.007
Lunardi G, Piccioli P, Bruzzi P, Notaro R, Lastraioli S, Serra M, Marroni P, Bighin C, Mansutti M, Puglisi F et al (2013) Plasma estrone sulfate concentrations and genetic variation at the CYP19A1 locus in postmenopausal women with early breast cancer treated with letrozole. Breast Cancer Res Treat 137:167–174. doi:10.1007/s10549-012-2306-z
Sini V, Lunardi G, Cirillo M, Turazza M, Bighin C, Giraudi S, Levaggi A, Piccioli P, Bisagni G, Gnoni R et al (2014) Body mass index and circulating oestrone sulphate in women treated with adjuvant letrozole. Br J Cancer 110:1133–1138. doi:10.1038/bjc.2014.2
Smith IE, Dowsett M, Yap YS, Walsh G, Lonning PE, Santen RJ, Hayes D (2006) Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 24:2444–2447. doi:10.1200/JCO.2005.05.3694
Acknowledgements
This study was supported in part by the National Institutes of Health [Grant Numbers U-01GM61373 (DAF), 5T32-GM08425 (DAF, JDR), M01-RR000042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU)], the Department of Defense [Grant Number W81XWH-10-1-0349 (JDR)], Pfizer (DFH), Novartis Pharma AG (DFH), and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale™ (DFH). Study medication was provided by Pfizer, Inc. and Novartis Pharma AG. We would like to thank the participating patients with breast cancer and the research nurse coordinators at each of the clinical trial sites. Finally, we express our gratitude to and respect for DAF, who provided tremendous expertise and leadership for this project prior to his untimely death.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
NLH received research funding from AstraZeneca, Eli Lilly, BioMarin Pharmaceuticals, Celldex Pharmaceuticals, and Sanofi Aventis. VS received research funding from Abbvie, Celgene, Medimmune, Merck, Novartis, Pfizer, and Puma. DFH worked as a consultant for Lilly Oncology, and received research funding from Merrimack, Lilly, Janssen R&D, Puma Biotechnology, Pfizer, and Astra Zeneca. DFH also has personal financial interest in Oncimmune and Inbiomotion and received royalties from Janssen R&D. DAF received research funding from Pfizer and Novartis and sat on the Scientific Board for Quest Diagnostics. The rest of the authors have no conflicts of interest to declare (JDR, ZD, ATN, LL, DLH, JMR, AMS, TCS).
Additional information
Todd C. Skaar, N. Lynn Henry: co-senior authors.
Rights and permissions
About this article
Cite this article
Robarge, J.D., Desta, Z., Nguyen, A.T. et al. Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer. Breast Cancer Res Treat 161, 453–461 (2017). https://doi.org/10.1007/s10549-016-4077-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4077-4