Abstract
Purpose
The randomized phase III JO21095 trial compared the efficacy and safety of low-dose capecitabine plus docetaxel combination therapy (XT) versus single-agent administration of docetaxel in anthracycline-pretreated HER2-negative metastatic breast cancer.
Methods
Patients were randomized to either low-dose XT (capecitabine 825 mg/m2 twice daily, days 1–14; docetaxel 60 mg/m2, day 1 every 3 weeks) or docetaxel (70 mg/m2, day 1 every 3 weeks). The primary objective was to demonstrate superior progression-free survival (PFS) with low-dose XT versus single-agent docetaxel. Overall survival (OS) and safety were secondary endpoints.
Results
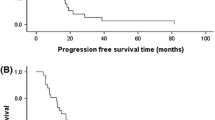
In total, 162 patients were treated. Median PFS was 10.5 months with low-dose XT and 9.8 months with single-agent docetaxel (hazard ratio [HR] 0.62 [95% confidence interval (CI) 0.40–0.97]; p = 0.03). The OS HR was 0.89 (95% CI 0.52–1.53; p = 0.68). Grade ≥3 treatment-related toxicities occurred in 74% of XT-treated patients and 76% of docetaxel-treated patients. The main differences in grade ≥3 treatment-related toxicities were hand-foot syndrome (7.3% of XT-treated patients vs 0% receiving docetaxel), fatigue/malaise (2.4 vs 10.0%), and peripheral edema (1.2 vs 7.5%). Dose modifications were required in 100% of low-dose XT and 49% of docetaxel patients. Toxicity-related treatment discontinuations occurred in 18 and 33%, respectively.
Conclusion
The improved PFS with low-dose XT versus docetaxel alone is consistent with higher-dose XT phase III experience, but the safety profile was more favorable and manageable.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
De Angelis R, Sant M, Coleman MP, EUROCARE-5 Working Group et al (2014) Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol 15:23–34
Verma S, McLeod D, Batist G, Robidoux A, Martins IR, Mackey JR (2011) In the end what matters most? A review of clinical endpoints in advanced breast cancer. Oncologist 16:25–35
O’Shaughnessy J, Miles D, Vukelja S et al (2002) Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 20:2812–2823
Beslija S, Obralic N, Basic H et al (2006) Randomized trial of sequence vs. combination of capecitabine (X) and docetaxel (T): XT vs. T followed by X after progression as first-line therapy for patients (pts) with metastatic breast cancer (MBC). J Clin Oncol 24(18 suppl):571 (Abstract)
Leonard R, O’Shaughnessy J, Vukelja S et al (2006) Detailed analysis of a randomized phase III trial: can the tolerability of capecitabine plus docetaxel be improved without compromising its survival advantage? Ann Oncol 17:1379–1385
Leonard R, Hennessy BT, Blum JL, O’Shaughnessy J (2011) Dose-adjusting capecitabine minimizes adverse effects while maintaining efficacy: a retrospective review of capecitabine for metastatic breast cancer. Clin Breast Cancer 11:349–356
Hennessy BT, Gauthier AM, Michaud LB, Hortobagyi G, Valero V (2005) Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at M. D. Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol 16:1289–1296
Barrios CH, Liu MC, Lee SC et al (2010) Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 121:121–131
Robert NJ, Dieras V, Glaspy J et al (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260
Stockler MR, Harvey VJ, Francis PA et al (2011) Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 29:4498–4504
Thomas ES, Gomez HL, Li RK et al (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210–5217
Zielinski C, Gralow J, Martin M (2010) Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol 21:2145–2152
Buzdar AU, Xu B, Digumarti R et al (2012) Randomized phase II non-inferiority study (NO16853) of two different doses of capecitabine in combination with docetaxel for locally advanced/metastatic breast cancer. Ann Oncol 23:589–597
Aogi K, Saeki T, Minami H et al (2007) The Japanese Breast Cancer Society (Abstract O-268)
Brookmeyer R, Crowley JJ (1982) A confidence interval for the median survival time. Biometrics 38:29–41
Bachelot T, Bajard A, Ray-Coquard I et al (2011) Final results of ERASME-4: a randomized trial of first-line docetaxel plus either capecitabine or epirubicin for metastatic breast cancer. Oncology 80:262–268
Mavroudis D, Papakotoulas P, Ardavanis A et al (2010) Randomized phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first-line treatment in women with advanced breast cancer. Ann Oncol 21:48–54
Acknowledgements
The authors wish to thank the patients and their families, study site personnel, and the study team for their participation in the trial. The JO21095 study was conducted by Chugai Pharmaceutical to fulfill a post-marketing commitment. Chugai Pharmaceutical was involved in the study design, statistical analyses, and data interpretation. Chugai Clinical Research Center Co., Ltd., gathered and managed data, and Quintiles Transnational Japan K. K. was responsible for data monitoring. The principal investigators (IH, NM, and KK) had full access to all study data and had final responsibility for the publication. Chugai funded third-party writing assistance for this manuscript and provided by Jennifer Kelly (Medi-Kelsey Ltd, UK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y Yamamoto has received honoraria from Chugai Pharmaceutical; J. Watanabe received remuneration for speaker engagements from Chugai Pharmaceutical and Eizai; N. Masuda has received honoraria from Chugai Pharmaceutical, Astra-Zeneca and Kyowa Hakko Kirin; H. Iwata has received honoraria from Chugai Pharmaceutical and has served as a consultant or advisory role for Chugai Pharmaceutical; M. Saito has received funding from Eizai; S. Hattori has served as a consultant or advisory role for Chugai Pharmaceutical; D. Yamamoto, N. Sato, Y. Rai, S. Oura, Y. Matsuura, and K. Kuroi declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The ethical committee approved the study protocol, and all patients provided written informed consent before undergoing any study-specific procedure. The trial complies with current Japanese laws.
Rights and permissions
About this article
Cite this article
Yamamoto, D., Sato, N., Rai, Y. et al. Efficacy and safety of low-dose capecitabine plus docetaxel versus single-agent docetaxel in patients with anthracycline-pretreated HER2-negative metastatic breast cancer: results from the randomized phase III JO21095 trial. Breast Cancer Res Treat 161, 473–482 (2017). https://doi.org/10.1007/s10549-016-4075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4075-6