Abstract
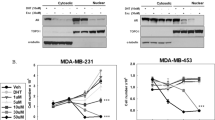
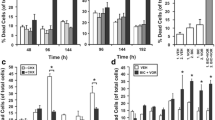
Triple-negative breast cancers (TNBC) are aggressive malignancies with no effective targeted therapies. Recent gene expression profiling of these heterogeneous cancers and the classification of cell line models now allows for the identification of compounds with selective activities against molecular subtypes of TNBC. The natural product deguelin was found to have selective activity against MDA-MB-453 and SUM-185PE cell lines, which both model the luminal androgen receptor (LAR) subtype of TNBC. Deguelin potently inhibited proliferation of these cells with GI50 values of 30 and 61 nM, in MDA-MB-453 and SUM-185PE cells, respectively. Deguelin had exceptionally high selectivity, 197 to 566-fold, for these cell lines compared to cell lines representing other TNBC subtypes. Deguelin’s mechanisms of action were investigated to determine how it produced these potent and selective effects. Our results show that deguelin has dual activities, inhibiting PI3K/Akt/mTOR signaling, and decreasing androgen receptor levels and nuclear localization. Based on these data, we hypothesized that the combination of the mTOR inhibitor rapamycin and the antiandrogen enzalutamide would have efficacy in LAR models. Rapamycin and enzalutamide showed additive effects in MDA-MB-453 cells, and both drugs had potent antitumor efficacy in a LAR xenograft model. These results suggest that the combination of antiandrogens and mTOR inhibitors might be an effective strategy for the treatment of androgen receptor-expressing TNBC.






Similar content being viewed by others
References
Irvin WJ, Carey LA (2008) What is triple-negative breast cancer? Eur J Cancer 44:2799–2805. doi:10.1016/j.ejca.2008.09.034
Venkitaraman R (2010) Triple-negative/basal-like breast cancer: clinical, pathologic and molecular features. Expert Rev Anticancer Ther 10:199–207. doi:10.1586/era.09.189
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752. doi:10.1038/35021093
Perou CM (2011) Molecular stratification of triple-negative breast cancers. The Oncologist 16(Suppl 1):61–70. doi:10.1634/theoncologist.2011-S1-61
Vaz-Luis I, Ottesen RA, Hughes ME et al (2014) Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 32:2142–2150. doi:10.1200/JCO.2013.53.1608
Yu K-D, Zhu R, Zhan M et al (2013) Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin Cancer Res 19:2723–2733. doi:10.1158/1078-0432.CCR-12-2986
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Liu NQ, Stingl C, Look MP et al (2014) Comparative proteome analysis revealing an 11-protein signature for aggressive triple-negative breast cancer. J Natl Cancer Inst. doi:10.1093/jnci/djt376
Howlader N, Altekruse SF, Li CI et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status 106: dju055. J Natl Cancer Inst. doi:10.1093/jnci/dju055
Huo D, Ikpatt F, Khramtsov A et al (2009) Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 27:4515–4521. doi:10.1200/JCO.2008.19.6873
Amirikia KC, Mills P, Bush J, Newman LA (2011) Higher population-based incidence rates of triple-negative breast cancer among young African-American women: implications for breast cancer screening recommendations. Cancer 117:2747–2753. doi:10.1002/cncr.25862
Brewster AM, Chavez-MacGregor M, Brown P (2014) Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 15:e625–e634. doi:10.1016/S1470-2045(14)70364-X
Clarke CA, Keegan THM, Yang J et al (2012) Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 104:1094–1101. doi:10.1093/jnci/djs264
Iqbal J, Ginsburg O, Rochon PA et al (2015) Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313:165–173. doi:10.1001/jama.2014.17322
Bonifazi M, Franchi M, Rossi M et al (2014) Long term survival of HER2-positive early breast cancer treated with trastuzumab-based adjuvant regimen: a large cohort study from clinical practice. The Breast 23:573–578. doi:10.1016/j.breast.2014.05.022
Gámez-Pozo A, Pérez Carrión RM, Manso L et al (2014) The long-HER study: clinical and molecular analysis of patients with HER2 + advanced breast cancer who become long-term survivors with trastuzumab-based therapy. PLoS One 9:e109611. doi:10.1371/journal.pone.0109611
Brower V (2014) Adjuvant trastuzumab improves survival in early breast cancer. Lancet Oncol 15:e589. doi:10.1016/S1470-2045(14)71110-6
Baselga J, Perez EA, Pienkowski T, Bell R (2006) Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncol 11(Suppl 1):4–12. doi:10.1634/theoncologist.11-90001-4
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New Engl J Med 353:1673–1684. doi:10.1056/NEJMoa052122
Davies C, Godwin J, Gray R, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. doi:10.1016/S0140-6736(11)60993-8
Marques RB, Aghai A, de Ridder CMA et al (2014) High efficacy of combination therapy using PI3K/AKT inhibitors with androgen deprivation in prostate cancer preclinical models. Eur Urol 67:1177–1185. doi:10.1016/j.eururo.2014.08.053
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. doi:10.1016/S0140-6736(05)66544-0
Bosch A, Eroles P, Zaragoza R et al (2010) Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 36:206–215. doi:10.1016/j.ctrv.2009.12.002
Hirshfield KM, Ganesan S (2014) Triple-negative breast cancer: molecular subtypes and targeted therapy. Curr Opin Obstet Gynecol 26:34–40. doi:10.1097/GCO.0000000000000038
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA (2014) New strategies for triple-negative breast cancer—deciphering the heterogeneity. Clin Cancer Res 20:782–790. doi:10.1158/1078-0432.CCR-13-0583
Abramson VG, Mayer IA (2014) Molecular heterogeneity of triple negative breast cancer. Curr Breast Cancer Rep 6:154–158. doi:10.1007/s12609-014-0152-1
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121:2750–2767. doi:10.1172/JCI45014
Kreike B, van Kouwenhove M, Horlings H et al (2007) Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res 9:R65. doi:10.1186/bcr1771
Lehmann BD, Bauer JA, Schafer JM et al (2014) PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 16:406. doi:10.1186/s13058-014-0406-x
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335. doi:10.1021/np200906s
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim et Biophys Acta 1830:3670–3695. doi:10.1016/j.bbagen.2013.02.008
Patridge E, Gareiss P, Kinch MS, Hoyer D (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21:204–207. doi:10.1016/j.drudis.2015.01.009
Mann J (2002) Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2:143–148. doi:10.1038/nrc723
Newman DJ, Cragg GM (2009) Natural product scaffolds as leads to drugs. Future Med Chem 1:1415–1427. doi:10.4155/fmc.09.113
Boyd MR, Paull KD, Rubinstein LR (1992) Data display and analysis strategies for the NCI disease-oriented in vitro antitumor drug screen. Cytotoxic anticancer. Drugs: model. Concepts Drug Discov. Dev. Springer US, pp 11–34
Skehan P, Storeng R, Scudiero D et al (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Yenesew A, Mushibe EK, Induli M et al (2005) 7a-O-methyldeguelol, a modified rotenoid with an open ring-C, from the roots of Derris trifoliata. Phytochemistry 66:653–657. doi:10.1016/j.phytochem.2005.02.005
Clark EP (1931) A relation between rotenone, deguelin and tephrosin. Science 73:17–18. doi:10.1126/science.73.1879.17
Delfel NE, Tallent WH, Carlson DG, Wolff IA (1970) Distribution of rotenone and deguelin in Tephrosia vogelii and separation of rotenoid-rich fractions. J Agric Food Chem 18:385–390
Ashack RJ, McCarty LP, Malek RS et al (1980) Evaluation of rotenone and related compounds as antagonists of slow-reacting substance of anaphylaxis. J Med Chem 23:1022–1026. doi:10.1021/jm00183a011
Xu H, Li X, Ding W et al (2015) Deguelin induces the apoptosis of lung cancer cells through regulating a ROS driven Akt pathway. Cancer Cell Int 15:25. doi:10.1186/s12935-015-0166-4
Baba Y, Fujii M, Maeda T et al (2015) Deguelin induces apoptosis by targeting both EGFR-Akt and IGF1R-Akt pathways in head and neck squamous cell cancer cell lines. Biomed Res Int 2015:657179. doi:10.1155/2015/657179
O’Reilly KE, Rojo F, She Q-B et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508. doi:10.1158/0008-5472.CAN-05-2925
Oh SH, Woo JK, Yazici YD et al (2007) Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst 99:949–961. doi:10.1093/jnci/djm007
Moore NL, Buchanan G, Harris JM et al (2012) An androgen receptor mutation in the MDA-MB-453 cell line model of molecular apocrine breast cancer compromises receptor activity. Endocr Relat Cancer 19:599–613. doi:10.1530/ERC-12-0065
Cochrane DR, Bernales S, Jacobsen BM et al (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16:R7. doi:10.1186/bcr3599
Lee S-C, Min H-Y, Choi H et al (2015) Synthesis and evaluation of a novel deguelin derivative, L80, which disrupts ATP binding to the C-terminal domain of heat shock protein 90. Mol Pharm 88:245–255. doi:10.1124/mol.114.096883
Barton VN, D’Amato NC, Gordon MA et al (2015) Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther 14:769–778. doi:10.1158/1535-7163.MCT-14-0926
Lea OA, Kvinnsland S, Thorsen T (1989) Improved measurement of androgen receptors in human breast cancer. Cancer Res 49:7162–7167
Gucalp A, Tolaney S, Isakoff SJ, Traina et al (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 19:5505–5512. doi:10.1158/1078-0432.CCR-12-3327
Ellard SL, Clemons M, Gelmon KA et al (2009) Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol 27:4536–4541. doi:10.1200/JCO.2008.21.3033
Elkabets M, Vora S, Juric D et al (2013) mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 5:196ra99. doi:10.1126/scitranslmed.3005747
Eccles DM, Vachon CM, Couch FJ, Purrington KS, Visscher DW, Wang C, Mannermaa et al (2016) Genes associated with histopathologic features of triple negative breast tumors predict molecular subtypes. Breast Cancer Res Treat. doi:10.1007/s10549-016-3775-2
Acknowledgments
This study was funded by grants to S.L.M. and R.H.C. from the National Cancer Institute (UO1CA182740), the UTHSCSA President’s Council Excellence Award (S.L.M), and the Greehey Distinguished Chair in Targeted Molecular Therapeutics endowment (S.L.M.). Support of the Flow Cytometry, Macromolecular Structure and Mass Spectrometry Shared Resources of the CTRC Cancer Center Support Grant (P30 CA054174) are gratefully acknowledged. A.J.R was partially supported by the IMSD program of the NIGMS (1R25GM095480-01). The authors would like to thank the San Antonio Botanical Gardens for allowing us to collect samples of Amorpha fruticosa used in this study. We thank Dr. April Risinger for her thoughtful review of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robles, A.J., Cai, S., Cichewicz, R.H. et al. Selective activity of deguelin identifies therapeutic targets for androgen receptor-positive breast cancer. Breast Cancer Res Treat 157, 475–488 (2016). https://doi.org/10.1007/s10549-016-3841-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3841-9