Abstract
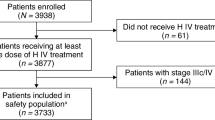
We critically examined long-term cardiovascular (CV) outcomes and overall survival (OS) of breast cancer (BC) patients who had cardiotoxicity during adjuvant trastuzumab treatment requiring discontinuation in a population-based sample. This was a retrospective cohort of early-stage BC patients diagnosed before 2010 and treated with trastuzumab in Ontario. Patients were stratified based on trastuzumab doses received: 1–8, 9–15, ≥16 (therapy completion). Time-dependent multivariable Cox models were used to analyze primary endpoint OS, and the following composite endpoints: hospitalization/emergency room visit for heart failure (HF) or death; non-HF CV (myocardial infarction, stroke) or death; and clinically significant relapse (palliative systemic therapy initiation >90 days after last trastuzumab dose) or death. Of the 3134 women, 6, 10, and 85 % received 1–8, 9–15, and ≥16 doses, respectively. Over 5-year median follow-up, early trastuzumab discontinuation was associated with more HF/death [1–8 doses hazard ratio (HR) 4.0, 95 % confidence interval (CI) 2.7–6.0; 9–15 doses HR 2.97, 95 % CI 2.1–4.3], non-HF/death (1–8 doses HR 4.3, 95 % CI 3.0–6.1; 9–15 doses HR 3.1, 95 % CI 2.2–4.4), clinically significant relapse/death (1–8 doses HR 3.1, 95 % CI 2.2–4.4; 9–15 doses HR 2.4, 95 % CI 1.8–3.3), and importantly lower OS (77, 80, 93 %; P < 0.001). Early discontinuation (1–8 doses HR 2.41, 95 % CI 1.5–3.8; 9–15 doses HR 2.9, 95 % CI 2.0–4.1) and clinically significant relapse (HR 34.0, 95 % CI 24.9–46.6) were both independent predictors of mortality. Of note, early discontinuation remained a critical independent predictor of OS even after adjusting for incident HF. Early trastuzumab discontinuation is a powerful independent predictor of cardiac events and clinically significant relapse, and both may contribute to poor survival. Both adequate cancer control and optimal CV management are required to improve long-term outcomes.


Similar content being viewed by others
References
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D’Amico R (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 4:CD006243
Yeh ET, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53(24):2231–2247
Truong J, Yan AT, Cramarossa G, Chan KK (2014) Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol 30(8):869–878
de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, Untch M, Smith IE, Gianni L, Baselga J et al (2014) Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol Off J Am Soc Clin Oncol 32(20):2159–2165
Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN et al (2008) Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol Off J Am Soc Clin Oncol 26(8):1231–1238
Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA et al (2012) Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 30(31):3792–3799
Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L et al (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol Off J Am Soc Clin Oncol 23(31):7811–7819
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I et al (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141
Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore I Jr, Lindquist DL, Holmes FA, Allison MA, Brooks BD et al (2013) Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol 14(11):1121–1128
Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL et al (2012) Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 104(17):1293–1305
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP (2012) Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 60(24):2504–2512
Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN, Giordano SH (2013) Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 31(33):4222–4228
McArthur HL, Chia S (2007) Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med 357(1):94–95
Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, Lestuzzi C, Maurea N, Oliva S, Russo G et al (2012) Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Cardiac Fail 18(2):113–119
Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, Ceccherini R, Bovelli D, Lestuzzi C, Cioffi G et al (2012) Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol Off J Eur Soc Med Oncol 23(12):3058–3063
Du XL, Xia R, Burau K, Liu CC (2011) Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998–2005. Med Oncol 28(Suppl 1):S80–S90
Telli ML, Hunt SA, Carlson RW, Guardino AE (2007) Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol Off J Am Soc Clin Oncol 25(23):3525–3533
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64(4):252–271
Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K (2011) Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol Off J Am Soc Clin Oncol 29(30):4014–4021
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619
Schultz SE, Rothwell DM, Chen Z, Tu K (2013) Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 33(3):160–166
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34(28):3661–3679
Onitilo AA, Engel JM, Stankowski RV (2014) Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf 5(4):154–166
Ho KK, Pinsky JL, Kannel WB, Levy D (1993) The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 22(4 Suppl A):6A–13A
Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL et al (2007) Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 116(13):1482–1487
Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, Kentepozidis N, Ziras N, Georgoulias V, Breast Cancer Investigators of the Hellenic Oncology Research Group AG (2015) Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol Off J Eur Soc Med Oncol 26(7):1333–1340
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D et al (2013) 6 Months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 14(8):741–748
Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, Chen J (2014) Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat 146(2):411–419
Perez EA, Rodeheffer R (2004) Clinical cardiac tolerability of trastuzumab. J Clin Oncol Off J Am Soc Clin Oncol 22(2):322–329
Ewer MS, Lippman SM (2005) Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol Off J Am Soc Clin Oncol 23(13):2900–2902
Ewer MS, Ewer SM (2015) The anthracycline–trastuzumab interaction: a lesson in not jumping to confusion. Trends Pharmacol Sci 36(6):321–322
Bria E, Cuppone F, Fornier M, Nistico C, Carlini P, Milella M, Sperduti I, Terzoli E, Cognetti F, Giannarelli D (2008) Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat 109(2):231–239
Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M et al (2010) Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol Off J Am Soc Clin Oncol 28(21):3422–3428
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW (2010) Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol Off J Am Soc Clin Oncol 28(21):3416–3421
Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, Marks L, Davidson N, Martino S, Kaufman P et al (2009) Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol Off J Am Soc Clin Oncol 27(16):2638–2644
D’Agostino RB Jr, D’Agostino RB Sr (2007) Estimating treatment effects using observational data. JAMA 297(3):314–316
Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K, Rugo H, Miller K, Ellis M, Shapira I et al (2016) Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2(1):29–36
Lenihan DJ (2014) Cardiac biomarkers, cardiotoxicity, and active collaboration: Is this the final frontier or the wave we should catch? J Am Coll Cardiol 63(8):817–818
Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA et al (2010) Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol Off J Am Soc Clin Oncol 28(25):3910–3916
Keefe DL (2002) Trastuzumab-associated cardiotoxicity. Cancer 95(7):1592–1600
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol Off J Am Soc Clin Oncol 20(5):1215–1221
Zeglinski M, Ludke A, Jassal DS, Singal PK (2011) Trastuzumab-induced cardiac dysfunction: a ‘dual-hit’. Exp Clin Cardiol 16(3):70–74
Goldhar HA, Yan AT, Ko DT, Earle CC, Tomlinson GA, Trudeau ME, Krahn MD, Krzyzanowska MK, Pal RS, Brezden-Masley C et al (2016) The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst 108(1):djv301
Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128(16):e240–e327
Funding
This work was supported by the Canadian Cancer Society (Grant #700839). This research was supported through provision of data by ICES and Cancer Care Ontario (CCO) and through funding support to ICES from an Annual Grant by the Ministry of Health and Long-Term Care (MOHLTC) and the Ontario Institute for Cancer Research (OICR). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, CCO, OICR or the Government of Ontario is intended or should be inferred.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, I.Y., Verma, S., Yan, A.T. et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat 157, 535–544 (2016). https://doi.org/10.1007/s10549-016-3823-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3823-y