Abstract
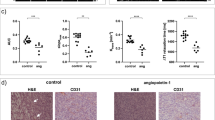
To employ in vivo imaging and histological techniques to identify and quantify vascular changes early in the course of treatment with trastuzumab in a murine model of HER2+ breast cancer. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was used to quantitatively characterize vessel perfusion/permeability (via the parameter K trans) and the extravascular extracellular volume fraction (v e ) in the BT474 mouse model of HER2+ breast cancer (N = 20) at baseline, day one, and day four following trastuzumab treatment (10 mg/kg). Additional cohorts of mice were used to quantify proliferation (Ki67), microvessel density (CD31), pericyte coverage (α-SMA) by immunohistochemistry (N = 44), and to quantify human VEGF-A expression (N = 29) throughout the course of therapy. Longitudinal assessment of combination doxorubicin ± trastuzumab (N = 42) tested the hypothesis that prior treatment with trastuzumab will increase the efficacy of subsequent doxorubicin therapy. Compared to control tumors, trastuzumab-treated tumors exhibited a significant increase in K trans (P = 0.035) on day four, indicating increased perfusion and/or vessel permeability and a simultaneous significant increase in v e (P = 0.01), indicating increased cell death. Immunohistochemical and ELISA analyses revealed that by day four the trastuzumab-treated tumors had a significant increase in vessel maturation index (i.e., the ratio of α-SMA to CD31 staining) compared to controls (P < 0.001) and a significant decrease in VEGF-A (P = 0.03). Additionally, trastuzumab dosing prior to doxorubicin improved the overall effectiveness of the therapies (P < 0.001). This study identifies and validates improved perfusion characteristics following trastuzumab therapy, resulting in an improvement in trastuzumab-doxorubicin combination therapy in a murine model of HER2+ breast cancer. This data suggests properties of vessel maturation. In particular, the use of DCE-MRI, a clinically available imaging method, following treatment with trastuzumab may provide an opportunity to optimize the scheduling and improve delivery of subsequent cytotoxic therapy.







Similar content being viewed by others
Abbreviations
- α-SMA:
-
Alpha- smooth muscle actin
- ANOVA:
-
Analysis of variance
- BCA:
-
Bicinchoninic
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- ELISA:
-
Enzyme-linked immunosorbent assay
- Gd-DTPA:
-
Gadolinium-diethylenetriaminepentaacetic acid
- HER2+:
-
Human epidermal growth factor receptor 2 positive
- H&E:
-
Hematoxylin and eosin stain
- MAPK:
-
Mitogen-activated protein kinase
- MVD:
-
Microvessel density
- NEX:
-
Number of excitations
- PI3K/AKT:
-
Phosphoinositide 3-kinase/protein kinase B
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region-of-interest
- TE:
-
Echo time
- TR:
-
Repetition time
- VEGF:
-
Vascular endothelial growth factor
- VMI:
-
Vessel maturation index
References
Mitri Z, Constantine T, O’Regan R (2012) The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract 2012:743193
Dean-Colomb W, Esteva FJ (2008) Her2-positive breast cancer: herceptin and beyond. Eur J Cancer 44(18):2806–2812
Spector NL, Blackwell KL (2009) Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 27(34):5838–5847
Nahta R, Esteva FJ (2006) HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res (BCR) 8(6):215
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421(6924):756–760
Menard S, Pupa SM, Campiglio M, Tagliabue E (2003) Biologic and therapeutic role of HER2 in cancer. Oncogene 22(42):6570–6578
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2:62
Klapper LN, Kirschbaum MH, Sela M, Yarden Y (2000) Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res 77:25–79
Zhang A, Shen G, Zhao T, Zhang G, Liu J, Song L, Wei W, Bing L, Wu Z, Wu Q (2010) Augmented inhibition of angiogenesis by combination of HER2 antibody chA21 and trastuzumab in human ovarian carcinoma xenograft. J Ovarian Res 3:20
Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416(6878):279–280
Klos KS, Zhou X, Lee S, Zhang L, Yang W, Nagata Y, Yu D (2003) Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer 98(7):1377–1385
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Hortobagyi GN (2005) Trastuzumab in the treatment of breast cancer. N Engl J Med 353(16):1734–1736
Jain RK, Carmeliet P (2012) SnapShot: tumor angiogenesis. Cell 149 (6):1408–1408 e1
Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31(17):2205–2218
Goel S, Wong AH, Jain RK (2012) Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2(3):a006486
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91(3):1071–1121
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989. doi:10.1038/nm0901-987
Yankeelov TE, Gore JC (2009) Dynamic contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr Med Imaging Rev 3(2):91–107
Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, Farley J, Mayer IA, Kelley MC, Meszoely IM, Means-Powell J, Grau AM, Sanders M, Yankeelov TE (2015) Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol 50(4):195–204
Andersen EK, Hole KH, Lund KV, Sundfor K, Kristensen GB, Lyng H, Malinen E (2013) Pharmacokinetic parameters derived from dynamic contrast enhanced MRI of cervical cancers predict chemoradiotherapy outcome. Radiother Oncol 107(1):117–122
Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, Weatherall PT, Lehman CD, Newstead GM, Polin S, Marques HS, Esserman LJ, Schnall MD, Team AT, Investigators IST (2012) Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology 263(3):663–672
Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Horita Y, Akiyoshi K, Takashima A, Okita N, Takahari D, Nakajima T, Hamaguchi T, Shimada Y, Shirao K (2012) Pharmacokinetic parameters from 3-Tesla DCE-MRI as surrogate biomarkers of antitumor effects of bevacizumab plus FOLFIRI in colorectal cancer with liver metastasis. Int J Cancer 130(10):2359–2365
O’Connor JP, Jackson A, Parker GJ, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96(2):189–195
Barnes SL, Whisenant JG, Loveless ME, Ayers GD, Yankeelov TE (2013) Assessing the reproducibility of dynamic contrast enhanced magnetic resonance imaging in a murine model of breast cancer. Magn Reson Med 69(6):1721–1734
Barnes SL, Whisenant JG, Loveless ME, Yankeelov TE (2012) Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics 4(3):442–478
Wu X, Jeong EK, Emerson L, Hoffman J, Parker DL, Lu ZR (2010) Noninvasive evaluation of antiangiogenic effect in a mouse tumor model by DCE-MRI with Gd-DTPA cystamine copolymers. Mol Pharm 7(1):41–48
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J (1998) Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58(13):2825–2831
Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM (1993) Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother (CII) 37(4):255–263
Whisenant JG, McIntyre JO, Peterson TE, Kang H, Sanchez V, Manning HC, Arteaga CL, Yankeelov TE (2014) Utility of [F]FLT-PET to assess treatment response in trastuzumab-resistant and trastuzumab-sensitive HER2-overexpressing human breast cancer xenografts. Mol Imaging Biol (MIB) 17(1):119–128
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10(6):515–527
Whisenant JG, Sorace AG, McIntyre JO, Kang H, Sanchez V, Loveless ME, Yankeelov TE (2014) Evaluating treatment response using DW-MRI and DCE-MRI in trastuzumab responsive and resistant HER2-overexpressing human breast cancer xenografts. Transl Oncol 7(6):768–779
Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, Pauletti G, Powell JE, Pegram MD, Slamon DJ (2006) Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol 24(12):1831–1838
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging (JMRI) 10(3):223–232
Daldrup H, Shames DM, Wendland M, Okuhata Y, Link TM, Rosenau W, Lu Y, Brasch RC (1998) Correlation of dynamic contrast-enhanced magnetic resonance imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media. Pediatr Radiol 28(2):67–78
de Lussanet QG, Beets-Tan RG, Backes WH, van der Schaft DW, van Engelshoven JM, Mayo KH, Griffioen AW (2004) Dynamic contrast-enhanced magnetic resonance imaging at 1.5 Tesla with gadopentetate dimeglumine to assess the angiostatic effects of anginex in mice. Eur J Cancer 40(8):1262–1268
Vangestel C, Van de Wiele C, Van Damme N, Staelens S, Pauwels P, Reutelingsperger CP, Peeters M (2011) (99)mTc-(CO)(3) His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab. J Nucl Med 52(11):1786–1794. doi:10.2967/jnumed.111.092650
Ahmed S, Sami A, Xiang J (2015) HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer 22(2):101–116
Heyerdahl H, Roe K, Brevik EM, Dahle J (2013) Modifications in dynamic contrast-enhanced magnetic resonance imaging parameters after alpha-particle-emitting (2)(2)(7)Th-trastuzumab therapy of HER2-expressing ovarian cancer xenografts. Int J Radiat Oncol Biol Phys 87(1):153–159
McCormack DR, Walsh AJ, Sit W, Arteaga CL, Chen J, Cook RS, Skala MC (2014) In vivo hyperspectral imaging of microvessel response to trastuzumab treatment in breast cancer xenografts. Biomed Opt Express 5(7):2247–2261
Le XF, Mao W, Lu C, Thornton A, Heymach JV, Sood AK, Bast RC Jr (2008) Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle 7(23):3747–3758
Acknowledgments
We thank the National Cancer Institute for support through R01CA138599, P50CA098131, P30CA68485, R25CA092043, 5T32CA093240, and U01CA174706. We thank the Kleberg Foundation for the generous support of Vanderbilt's biomedical imaging program. The authors thank Dr. Carlos Arteaga for his guidance on the combination therapy portions of this project. Additionally, we would also like to thank Dr. Jin Chen, Dr. Shan Wang, Dr. Melissa Skala, and Ms. Amy Shah for their helpful conversations.
Authors’ contributions
AS and JW carried out DCE-MRI studies, AS and VS carried out histology studies. AS and AH performed the ELISA study. AS carried out the combination therapeutic studies. AS completed the statistical analysis. OM, CQ, AS, AH, JW, and TY were involved in the conception and design of the study. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interest to declare in relation to this manuscript.
Additional information
The following authors’ affliations have changed to The University of Texas at Austin: AGS (anna.sorace@austin.utexas.edu) and TEY (thomas.yankeelov@utexas.edu).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sorace, A.G., Quarles, C.C., Whisenant, J.G. et al. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: preliminary results. Breast Cancer Res Treat 155, 273–284 (2016). https://doi.org/10.1007/s10549-016-3680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3680-8