Abstract
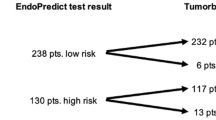
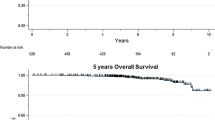
The purpose of this study was to evaluate the influence of guideline-based prospective use of uPA/PAI-1 on clinical outcome in an intermediate-risk cohort of breast cancer patients. We analyzed 381 consecutive primary breast cancer patients (2003–2011) at the breast center Ostbayern meeting the following criteria: M0/N0/estrogen receptor (ER)+/G2. Clinical–pathological data, uPA/PAI-1, and follow-up data were collected. Decisions for adjuvant chemotherapy were made upon consideration of prospectively measured uPA/PAI-1. Observed disease-free survival (DFS) and overall survival (OS) were calculated by Kaplan–Meier estimates. Using guideline-based analysis of uPA/PAI-1, treatment with adjuvant chemotherapy was avoided in 86.5 % of patients with low uPA/PAI-1, i.e., 38.8 % of the total patient collective. Median follow-up was 52.5 months. Five-year relapse-free survival in intermediate-risk patients (N0, G2) without chemotherapy was 99 %. Five-year overall survival including all causes of death was 95 %. By using uPA/PAI-1, adjuvant chemotherapy can be avoided in a major part of patients with intermediate-risk breast cancer. Nevertheless, DFS and OS of these patients at 5 years remain excellent. The potential, but hardly measurable, benefit of adjuvant chemotherapy has to be set in contrast with its associated side effects and increased morbidity. Patients with high uPA/PAI-1 show benefit from chemotherapy. In this subgroup, a very good OS was observed as well. These findings strongly support the use of uPA/PAI-1 together with clinic-pathological parameters as an evidence-based, clinically relevant and inexpensive decision tool in the routine of a breast center.




Similar content being viewed by others
References
Levi F, Bosetti C, Lucchini F, Negri E, La Vecchia C (2005) Monitoring the decrease in breast cancer mortality in Europe. Eur J Cancer Prev 14(6):497–502
Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK (2008) Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100(23):1672–1694. doi:10.1093/jnci/djn389
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/s0140-6736(05)66544-0
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ (2011) Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747. doi:10.1093/annonc/mdr304
Hanf V, Schutz F, Liedtke C, Thill M (2014) AGO recommendations for the diagnosis and treatment of patients with advanced and metastatic breast cancer: update 2014. Breast Care (Basel) 9(3):202–209. doi:10.1159/000363551
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. doi:10.1200/jco.2007.14.2364
Janicke F, Prechtl A, Thomssen C, Harbeck N, Meisner C, Untch M, Sweep CG, Selbmann HK, Graeff H, Schmitt M (2001) Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst 93(12):913–920
Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Ferno M, Eppenberger-Castori S, Sweep CG, Ulm K, Peyrat JP, Martin PM, Magdelenat H, Brunner N, Duggan C, Lisboa BW, Bendahl PO, Quillien V, Daver A, Ricolleau G, Meijer-van Gelder ME, Manders P, Fiets WE, Blankenstein MA, Broet P, Romain S, Daxenbichler G, Windbichler G, Cufer T, Borstnar S, Kueng W, Beex LV, Klijn JG, O’Higgins N, Eppenberger U, Janicke F, Schmitt M, Foekens JA (2002) Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst 94(2):116–128
Harbeck N, Schmitt M, Meisner C, Friedel C, Untch M, Schmidt M, Sweep CG, Lisboa BW, Lux MP, Beck T, Hasmuller S, Kiechle M, Janicke F, Thomssen C (2013) Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur J Cancer 49(8):1825–1835. doi:10.1016/j.ejca.2013.01.007
Harbeck N, Kates RE, Look MP, Meijer-Van Gelder ME, Klijn JG, Kruger A, Kiechle M, Janicke F, Schmitt M, Foekens JA (2002) Enhanced benefit from adjuvant chemotherapy in breast cancer patients classified high-risk according to urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (n = 3424). Cancer Res 62(16):4617–4622
Duffy MJ (2004) The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des 10(1):39–49
Schmitt M, Harbeck N, Brunner N, Janicke F, Meisner C, Muhlenweg B, Jansen H, Dorn J, Nitz U, Kantelhardt EJ, Thomssen C (2011) Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn 11(6):617–634. doi:10.1586/erm.11.47
Carriero MV, Stoppelli MP (2011) The urokinase-type plasminogen activator and the generation of inhibitors of urokinase activity and signaling. Curr Pharm Des 17(19):1944–1961
Janicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Hofler H, Graeff H (1993) Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat 24(3):195–208
Kantelhardt EJ, Vetter M, Schmidt M, Veyret C, Augustin D, Hanf V, Meisner C, Paepke D, Schmitt M, Sweep F, von Minckwitz G, Martin PM, Jaenicke F, Thomssen C, Harbeck N (2011) Prospective evaluation of prognostic factors uPA/PAI-1 in node-negative breast cancer: phase III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing 6xFEC versus 3xFEC/3xDocetaxel. BMC Cancer 11:140. doi:10.1186/1471-2407-11-140
Thomssen C, Kantelhardt EJ, Meisner C, Vetter M, Schmidt M, Martin PM, Veyret C, Augustin D, Hanf V, Paepke D, Meinerz W, Hoffmann G, Wiest W, Sweep FCGJ, Schmitt M, Jaenicke F, von Minckwitz G, Harbeck N (2012) First planned efficacy analysis of the NNBC3-Europe trial: addition of docetaxel to anthracycline containing adjuvant chemotherapy in high risk node-negative breast cancer patients. Cancer Res 72(24 Suppl):1–13
Jacobs VR, Kates RE, Kantelhardt E, Vetter M, Wuerstlein R, Fischer T, Schmitt M, Jaenicke F, Untch M, Thomssen C, Harbeck N (2013) Health economic impact of risk group selection according to ASCO-recommended biomarkers uPA/PAI-1 in node-negative primary breast cancer. Breast Cancer Res Treat 138(3):839–850. doi:10.1007/s10549-013-2496-z
Schmitt M, Mengele K, Gkazepis A, Napieralski R, Magdolen V, Reuning U, Harbeck N (2008) Assessment of urokinase-type plasminogen activator and its inhibitor PAI-1 in breast cancer tissue: historical aspects and future prospects. Breast Care (Basel) 3(s2):3–10. doi:10.1159/000151737
Acknowledgments
Statistical analysis (REK) was in part supported by the Breast Center of the University of Cologne.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This analysis was performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kolben, T., Augustin, D., Armbrust, R. et al. Impact of guideline-based use of uPA/PAI-1 on patient outcome in intermediate-risk early breast cancer. Breast Cancer Res Treat 155, 109–115 (2016). https://doi.org/10.1007/s10549-015-3653-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3653-3