Abstract
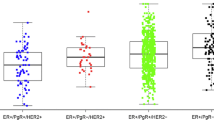
The 14-3-3ζ gene, on 8q22, is often amplified in breast cancer and encodes a survival factor that interacts with and stabilizes many key signaling proteins. We examined the relationship between the expression of 14-3-3ζ, estrogen receptor α (ERα), and other parameters ( tumor size, grade, nodal status, progesterone receptor, HER2, EGFR, and p53) in matched primary and recurrence tumor tissue and how these factors impact time to recurrence, properties of the recurred tumors, and site of metastasis. In this cohort of over 100 patients, median time to recurrence was 3 years (range 1–17 years). Our analyses of primary tumor microarray cores revealed that 14-3-3ζ status was significantly correlated with tumor grade, size, and ERα. Women with 14-3-3ζ-positive and ERα-negative tumors had the earliest time to recurrence (median 1 yr, p < 0.001, hazard ratio 2.89), while median time to recurrence was 7 years for 14-3-3ζ-negative and ER-positive tumors. Of recurred tumors, 70–75 % were positive for 14-3-3ζ, up from the 45 % positivity of primary tumors. High expression of 14-3-3ζ also correlated with site of recurrence and showed a propensity for distant metastases to lung and chest wall. Multifactor correlation regression analysis revealed 14-3-3ζ to be a non-redundant, independent variable that adds clinical strength in predicting risk for early recurrence in ER-positive and -negative breast cancers, providing information beyond that of all other clinical pathological features examined. Thus, high expression of 14-3-3ζ in the primary tumor was significantly associated with earlier time to recurrence and with distant metastasis. Furthermore, even when the primary breast cancers were negative-low for 14-3-3ζ, the majority acquired increased expression in the recurrence. The findings underscore the detrimental role played by 14-3-3ζ in tumor aggressiveness and suggest that reducing its expression or interfering with its actions might substantially improve the clinical outcome for breast cancer patients.




Similar content being viewed by others
Abbreviations
- ERα:
-
Estrogen receptor alpha
- E2:
-
Estradiol
- PgR:
-
Progesterone receptor
- Tam:
-
Tamoxifen
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Bergamaschi A, Christensen BL, Katzenellenbogen BS (2011) Reversal of endocrine resistance in breast cancer: interrelationships among 14–3-3zeta, FOXM1, and a gene signature associated with mitosis. Breast Cancer Res 13:R70
Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, Miller LD, Smeds J, Bergh J, Katzenellenbogen BS (2006) Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res 66:7334–7340
Tzivion G, Gupta VS, Kaplun L, Balan V (2006) 14–3-3 proteins as potential oncogenes. Semin Cancer Biol 16:203–213
Li Y, Zou L, Li Q, Haibe-Kains B, Tian R, Li Y, Desmedt C, Sotiriou C, Szallasi Z, Iglehart JD, Richardson AL, Wang ZC (2010) Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med 16:214–218
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365:671–679
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B, Ling C, Zhou X, Chen T, Chiao PJ, Feng X, Seewaldt VL, Muller WJ, Sahin A, Hung MC, Yu D (2009) 14–3-3zeta cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16:195–207
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan K-H, Ensor J, Hittelman W, Hung M-C, Yu D (2009) 14–3-3{zeta} overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 69:3425–3432
Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A (2004) Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem 279:25549–25561
Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindall DJ, Chen J (2008) Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol 10:1076–1082
McPherson RA, Harding A, Roy S, Lane A, Hancock JF (1999) Interactions of c-Raf-1 with phosphatidylserine and 14-3-3. Oncogene 18:3862–3869
Oksvold MP, Huitfeldt HS, Langdon WY (2004) Identification of 14-3-3[zeta] as an EGF receptor interacting protein. FEBS Lett 569:207–210
Bergamaschi A, Katzenellenbogen BS (2012) Tamoxifen downregulation of miR-451 increases 14-3-3zeta and promotes breast cancer cell survival and endocrine resistance. Oncogene 31:39–47
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor marker prognostic studies (remark). Exp Oncol 28:99–105
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA (2010) Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res 12:R92
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O’Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ (2000) Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124:966–978
Sawyers CL (2008) The cancer biomarker problem. Nature 452:548–552
Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE Jr, Wickerham DL, Wolmark N (2010) Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 28:1677–1683
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26:721–728
Ralhan R, Masui O, Desouza LV, Matta A, Macha M, Siu KW (2011) Identification of proteins secreted by head and neck cancer cell lines using LC-MS/MS: strategy for discovery of candidate serological biomarkers. Proteomics 11:2363–2376
Kobayashi R, Deavers M, Patenia R, Rice-Stitt T, Halbe J, Gallardo S, Freedman RS (2009) 14–3-3 zeta protein secreted by tumor associated monocytes/macrophages from ascites of epithelial ovarian cancer patients. Cancer Immunol Immunother 58:247–258
Danes CG, Wyszomierski SL, Lu J, Neal CL, Yang W, Yu D (2008) 14–3-3 zeta down-regulates p53 in mammary epithelial cells and confers luminal filling. Cancer Res 68:1760–1767
Liu ET, Karuturi KR (2004) Microarrays and clinical investigations. N Engl J Med 350:1595–1597
Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R, Hood L (2004) Proteomic analysis identifies that 14–3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci USA 101:15370–15375
Li Z, Zhao J, Du Y, Park HR, Sun S-Y, Bernal-Mizrachi L, Aitken A, Khuri FR, Fu H (2008) Down-regulation of 14–3-3ζ suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci USA 105:162–167
Acknowledgments
This research was supported by Grants from The Breast Cancer Research Foundation (B.S.K.), a Postdoctoral Fellowship from the Department of Defense (W81XWH-09-1-0398, A.B.), the Sirazi Breast Cancer Research Fund through the University of Illinois Cancer Center (B.S.K. and J.F.), and NIH (T32 HL07692, AS).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergamaschi, A., Frasor, J., Borgen, K. et al. 14-3-3ζ as a predictor of early time to recurrence and distant metastasis in hormone receptor-positive and -negative breast cancers. Breast Cancer Res Treat 137, 689–696 (2013). https://doi.org/10.1007/s10549-012-2390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2390-0