Abstract
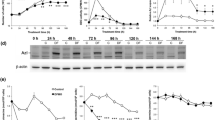
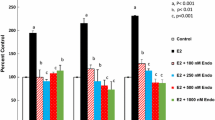
Our previous studies demonstrated that specific polyamine analogues, oligoamines, down-regulated the activity of a key polyamine biosynthesis enzyme, ornithine decarboxylase (ODC), and suppressed expression of estrogen receptor alpha (ERα) in human breast cancer cells. However, the mechanism underlying the potential regulation of ERα expression by polyamine metabolism has not been explored. Here, we demonstrated that RNAi-mediated knockdown of ODC (ODC KD) down-regulated the polyamine pool, and hindered growth in ERα-positive MCF7 and T47D and ERα-negative MDA-MB-231 breast cancer cells. ODC KD significantly induced the expression and activity of the key polyamine catabolism enzymes, spermine oxidase (SMO) and spermidine/spermine N 1-acetyltransferase (SSAT). However, ODC KD-induced growth inhibition could not be reversed by exogenous spermidine or overexpression of antizyme inhibitor (AZI), suggesting that regulation of ODC on cell proliferation may involve the signaling pathways independent of polyamine metabolism. In MCF7 and T47D cells, ODC KD, but not DFMO treatment, diminished the mRNA and protein expression of ERα. Overexpression of antizyme (AZ), an ODC inhibitory protein, suppressed ERα expression, suggesting that ODC plays an important role in regulation of ERα expression. Decrease of ERα expression by ODC siRNA altered the mRNA expression of a subset of ERα response genes. Our previous analysis showed that oligoamines disrupt the binding of Sp1 family members to an ERα minimal promoter element containing GC/CA-rich boxes. By using DNA affinity precipitation and mass spectrometry analysis, we identified ZBTB7A, MeCP2, PARP-1, AP2, and MAZ as co-factors of Sp1 family members that are associated with the ERα minimal promoter element. Taken together, these data provide insight into a novel antiestrogenic mechanism for polyamine biosynthesis enzymes in breast cancer.







Similar content being viewed by others
References
Porter CW, Herrera-Ornelas L, Pera P, Petrelli NF, Mittelman A (1987) Polyamine biosynthetic activity in normal and neoplastic human colorectal tissues. Cancer 60(6):1275–1281
LaMuraglia GM, Lacaine F, Malt RA (1986) High ornithine decarboxylase activity and polyamine levels in human colorectal neoplasia. Ann Surg 204(1):89–93
Manni A (2002) Polyamine involvement in breast cancer phenotype. In Vivo 16(6):493–500
Manni A (1994) The role of polyamines in the hormonal control of breast cancer cell proliferation. Cancer Treat Res 71:209–225
Thomas T, Thomas TJ (1994) Estradiol control of ornithine decarboxylase mRNA, enzyme activity, and polyamine levels in MCF-7 breast cancer cells: therapeutic implications. Breast Cancer Res Treat 29(2):189–201
Cohen FJ, Manni A, Glikman P, Bartholomew M, Demers L (1988) Involvement of the polyamine pathway in antiestrogen-induced growth inhibition of human breast cancer. Cancer Res 48(23):6819–6825
Nemoto T, Hori H, Yoshimoto M, Seyama Y, Kubota S (2002) Overexpression of ornithine decarboxylase enhances endothelial proliferation by suppressing endostatin expression. Blood 99(4):1478–1481
Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ et al (2003) A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res 9(7):2769–2777
Huang Y, Keen JC, Pledgie A, Marton LJ, Zhu T, Sukumar S, Park BH, Blair B, Brenner K, Casero RA Jr et al (2006) Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells. J Biol Chem 281(28):19055–19063
Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ (1988) Synthetic polyamine analogues as antineoplastics. J Med Chem 31(6):1183–1190
Seely JE, Pegg AE (1983) Ornithine decarboxylase (mouse kidney). Methods Enzymol 94:158–161
Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 61(14):5370–5373
Huang Y, Stewart TM, Wu Y, Baylin SB, Marton LJ, Perkins B, Jones RJ, Woster PM, Casero RA Jr (2009) Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin Cancer Res 15(23):7217–7228
Mitchell JL, Leyser A, Holtorff MS, Bates JS, Frydman B, Valasinas AL, Reddy VK, Marton LJ (2002) Antizyme induction by polyamine analogues as a factor of cell growth inhibition. Biochem J 366(Pt 2):663–671
Mitchell JL, Simkus CL, Thane TK, Tokarz P, Bonar MM, Frydman B, Valasinas AL, Reddy VK, Marton LJ (2004) Antizyme induction mediates feedback limitation of the incorporation of specific polyamine analogues in tissue culture. Biochem J 384(Pt 2):271–279
Murai N, Murakami Y, Matsufuji S (2003) Identification of nuclear export signals in antizyme-1. J Biol Chem 278(45):44791–44798
Newman RM, Mobascher A, Mangold U, Koike C, Diah S, Schmidt M, Finley D, Zetter BR (2004) Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J Biol Chem 279(40):41504–41511
Huang Y, Keen JC, Hager E, Smith R, Hacker A, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Casero RA Jr et al (2004) Regulation of polyamine analogue cytotoxicity by c-Jun in human MDA-MB-435 cancer cells. Mol Cancer Res 2(2):81–88
Huang Y, Pledgie A, Rubin E, Marton LJ, Woster PM, Sukumar S, Casero RA Jr, Davidson NE (2005) Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells. Cancer Biol Ther 4(9):1006–1013
Huang Y, Pledgie A, Casero R Jr, Davidson N (2005) Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs 16(3):229–241
Zu X, Yu L, Sun Q, Liu F, Wang J, Xie Z, Wang Y, Xu W, Jiang Y (2009) SP1 enhances Zbtb7A gene expression via direct binding to GC box in HePG2 cells. BMC Res Notes 2:175
Choi WI, Jeon BN, Park H, Yoo JY, Kim YS, Koh DI, Kim MH, Kim YR, Lee CE, Kim KS et al (2008) Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN). J Biol Chem 283(43):29341–29354
Lee DK, Kang JE, Park HJ, Kim MH, Yim TH, Kim JM, Heo MK, Kim KY, Kwon HJ, Hur MW (2005) FBI-1 enhances transcription of the nuclear factor-kappaB (NF-kappaB)-responsive E-selectin gene by nuclear localization of the p65 subunit of NF-kappaB. J Biol Chem 280(30):27783–27791
Qu H, Qu D, Chen F, Zhang Z, Liu B, Liu H (2010) ZBTB7 overexpression contributes to malignancy in breast cancer. Cancer Invest 28(6):672–678
Wilson ME, Westberry JM (2009) Regulation of oestrogen receptor gene expression: new insights and novel mechanisms. J Neuroendocrinol 21(4):238–242
Westberry JM, Trout AL, Wilson ME (2009) Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology 151(2):731–740
Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE (2005) Release of methyl CpG binding proteins and histone deacetylase 1 from the estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol 19(7):1740–1751
Frizzell KM, Kraus WL (2009) PARP inhibitors and the treatment of breast cancer: beyond BRCA1/2? Breast Cancer Res 11(6):111
Babbar N, Gerner EW (2011) Targeting polyamines and inflammation for cancer prevention. Recent Results Cancer Res 188:49–64
Mamont PS, Duchesne MC, Grove J, Bey P (1978) Anti-proliferative properties of dl-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun 81(1):58–66
Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A (1986) Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep 70(7):843–845
Alhonen-Hongisto L, Poso H, Janne J (1980) Inhibition by derivatives of diguanidines of cell proliferation in Ehrlich ascites cells grown in cultures. Biochem J 188(2):491–501
Acknowledgments
This work was funded by NIH Grants CA 51085 and CA 98454, the Breast Cancer Research Foundation, Susan G. Komen Foundation and the Samuel and Emma Winters Foundation.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, Q., Jin, L., Casero, R.A. et al. Role of ornithine decarboxylase in regulation of estrogen receptor alpha expression and growth in human breast cancer cells. Breast Cancer Res Treat 136, 57–66 (2012). https://doi.org/10.1007/s10549-012-2235-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2235-x