Abstract
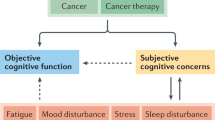
Chemotherapy-related cognitive impairment is a phenomenon of cognitive decline that some patients experience during and after chemotherapy. The prevalence of chemotherapy-related cognitive impairment in cancer survivors ranges from 14 to 85 %. Memory loss and lack of concentration are the most frequent symptoms, often resulting in deterioration of daily functioning and a decreased quality of life. Despite ongoing research on chemotherapy-related cognitive impairment, a clear understanding of the underlying mechanisms of the neurotoxicity induced by chemotherapy and the factors that determine a patient’s vulnerability are still lacking. We review current knowledge regarding the etiology of chemotherapy-related cognitive impairment, risk factors, conventional therapy, coping strategies, and potential complementary and integrative medicine treatments. Complementary and integrative medicine modalities that may improve chemotherapy-related cognitive impairment include mind–body techniques and acupuncture, as well as nutrition and herbal therapies. Studies on these modalities have not directly tested the hypothesis of modifying chemotherapy-related cognitive impairment and were done on different disorders of memory loss and lack of concentration. We recommend conducting further research on the potential role of complementary and integrative medicine modalities in the treatment and prevention of chemotherapy-related cognitive impairment.
Similar content being viewed by others
References
Nelson CJ, Nandy N, Roth AJ (2007) Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care 5:273–280
Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K (2005) Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients—evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer 12:279–287
Reid-Arndt SA (2009) Breast cancer and “chemobrain”: the consequences of cognitive difficulties following chemotherapy and the potential for recovery. Mo Med 106:127–131
Castellon S, Ganz PA (2009) Neuropsychological studies in breast cancer: in search of chemobrain. Breast Cancer Res Treat 116:125–127
Boykoff N, Moieni M, Subramanian SK (2009) Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 3:223–232
Whitney KA, Lysaker PH, Steiner AR, Hook JN, Estes DD, Hanna NH (2008) Is “chemobrain” a transient state? A prospective pilot study among persons with non-small cell lung cancer. J Support Oncol 6:313–321
Hede K (2008) Chemobrain is real but may need new name. J Natl Cancer Inst 100(162–163):169
Myers JS (2009) Chemotherapy-related cognitive impairment. Clin J Oncol Nurs 13:413–421
Dhillon H (2010) The fog hasn’t lifted on “chemobrain” yet: ongoing uncertainty regarding the effects of chemotherapy and breast cancer on cognition. Breast Cancer Res Treat 123:35–37
van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S (1998) Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210–218
Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS (2001) Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol 51:159–165
Ahles TA, Saykin AJ (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192–201
Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, Sheeder JL (1998) White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. AJNR Am J Neuroradiol 19:217–221
Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S (2012) Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 30:274–281
Li Y, Vijayanathan V, Gulinello M, Cole PD (2010) Intrathecal methotrexate induces focal cognitive deficits and increases cerebrospinal fluid homocysteine. Pharmacol Biochem Behav 95:428–433
Abraham A, Appaji L (2009) Cognitive assessment of children with acute lymphoblastic leukemia: preliminary findings. Indian J Med Paediatr Oncol 30:14–19
Madhyastha S, Somayaji SN, Rao MS, Nalini K, Bairy KL (2005) Effect of intracerebroventricular methotrexate on brain amines. Indian J Physiol Pharmacol 49:427–435
Riva D, Giorgi C, Nichelli F, Bulgheroni S, Massimino M, Cefalo G, Gandola L, Giannotta M, Bagnasco I, Saletti V, Pantaleoni C (2002) Intrathecal methotrexate affects cognitive function in children with medulloblastoma. Neurology 59:48–53
Verstappen CC, Heimans JJ, Hoekman K, Postma TJ (2003) Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 63:1549–1563
Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA (2007) Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem 100:191–201
Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, Estus S, Butterfield DA (2005) Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res 39:1147–1154
Joshi G, Hardas S, Sultana R, St Clair DK, Vore M, Butterfield DA (2007) Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: implication for chemobrain. J Neurosci Res 85:497–503
Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA (2010) Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience 166:796–807
Gounaris I, Ahmad A (2010) Capecitabine-induced cerebellar toxicity in a patient with metastatic colorectal cancer. J Oncol Pharm Pract 16:277–279
Mustafa S, Walker A, Bennett G, Wigmore PM (2008) 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci 28:230–323
Bradbury J (2006) Chemobrain: imaging shows changes in metabolism. Lancet Oncol 7:890
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA (2004) ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer 101:466–475
Hurria A, Somlo G, Ahles T (2007) Renaming “chemobrain”. Cancer Invest 25:373–377
Saykin AJ, Ahles TA, McDonald BC (2003) Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry 8:201–216
Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA (2007) Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat 103:303–311
Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB (2012) Cognitive functioning after cancer treatment: a 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer 118:1925–1932
Kohli S, Fisher SG, Tra Y, Adams MJ, Mapstone ME, Wesnes KA, Roscoe JA, Morrow GR (2009) The effect of modafinil on cognitive function in breast cancer survivors. Cancer 115:2605–2616
Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA (2007) Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology 16:772–777
Fitch MI, Armstrong J, Tsang S (2008) Patients’ experiences with cognitive changes after chemotherapy. Can Oncol Nurs J 18:180–192
Johnston MF, Yang C, Hui KK, Xiao B, Li XS, Rusiewicz A (2007) Acupuncture for chemotherapy-associated cognitive dysfunction: a hypothesis-generating literature review to inform clinical advice. Integr Cancer Ther 6:36–41
He J, Wu B, Zhang Y (2005) Acupuncture treatment for 15 cases of post-traumatic coma. J Tradit Chin Med 25:171–173
Allam H, ElDine NG, Helmy G (2008) Scalp acupuncture effect on language development in children with autism: a pilot study. J Altern Complement Med 14:109–114
Liu CZ, Yu JC, Zhang XZ, Fu WW, Wang T, Han JX (2006) Acupuncture prevents cognitive deficits and oxidative stress in cerebral multi-infarction rats. Neurosci Lett 393:45–50
Yu J, Liu C, Zhang X, Han J (2005) Acupuncture improved cognitive impairment caused by multi-infarct dementia in rats. Physiol Behav 86:434–441
Cheng H, Yu J, Jiang Z, Zhang X, Liu C, Peng Y, Chen F, Qu Y, Jia Y, Tian Q, Xiao C, Chu Q, Nie K, Kan B, Hu X, Han J (2008) Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett 432:111–116
Landin J, Frölich L, Schwarz S (2008) Use of alternative therapies in patients with dementia and mild cognitive impairment: a prospective, controlled study. Int J Geriatr Psychiatry 23:1163–1165
Iwasaki K, Kobayashi S, Chimura Y, Taguchi M, Inoue K, Cho S, Akiba T, Arai H, Cyong JC, Sasaki H (2004) A randomized, double-blind, placebo-controlled clinical trial of the Chinese herbal medicine “ba wei di huang wan” in the treatment of dementia. J Am Geriatr Soc 52:1518–1521
Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, Tanji H, Fujiwara H, Seki T, Fujii M, Arai H, Sasaki H (2005) A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry 66:248–252
Xu Y, Cao Z, Khan I, Luo Y (2008) Gotu Kola (Centella asiatica) extract enhances phosphorylation of cyclic AMP response element binding protein in neuroblastoma cells expressing amyloid beta peptide. J Alzheimers Dis 13:341–349
Arai H (2004) Current therapies in dementia. Nippon Ronen Igakkai Zasshi 41:310–313
Ryan J, Croft K, Mori T, Wesnes K, Spong J, Downey L, Kure C, Lloyd J, Stough C (2008) An examination of the effects of the antioxidant pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol 22:553–562
Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE (2000) Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil 81:668–678
Ernst E (2002) The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med 136:42–53
Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S (2005) Herb–drug interactions: a literature review. Drugs 65:1239–1282
Abebe W (2002) Herbal medication: potential for adverse interactions with analgesic drugs. J Clin Pharm Ther 27:391–401
Bebbington A, Kulkarni R, Roberts P (2005) Ginkgo biloba: persistent bleeding after total hip arthroplasty caused by herbal self-medication. J Arthroplasty 20:125–126
Hoerr R (2009) Ginkgo biloba does not reduce incidence of dementia in elderly people. Evid Based Ment Health 12:85
Parsons G (2009) Ginkgo biloba did not prevent dementia or Alzheimer disease in elderly people. Evid Based Nurs 12:56
DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Ginkgo Evaluation of Memory (GEM) Study Investigators (2008) Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA 300:2253–2262
van Dongen MC, van Rossum E, Kessels AG, Sielhorst HJ, Knipschild PG (2000) The efficacy of ginkgo for elderly people with dementia and age-associated memory impairment: new results of a randomized clinical trial. J Am Geriatr Soc 48:1183–1194
van Dongen M, van Rossum E, Kessels A, Sielhorst H, Knipschild P (2003) Ginkgo for elderly people with dementia and age-associated memory impairment: a randomized clinical trial. J Clin Epidemiol 56:367–376
Canter PH, Ernst E (2002) Ginkgo biloba: a smart drug? A systematic review of controlled trials of the cognitive effects of Ginkgo biloba extracts in healthy people. Psychopharmacol Bull 36:108–123
Canter PH, Ernst E (2008) Multiple n = 1 trials in the identification of responders and non-responders to the cognitive effects of Ginkgo biloba. Int J Clin Pharmacol Ther 41:354–357
Canter PH, Ernst E (2007) Ginkgo biloba is not a smart drug: an updated systematic review of randomised clinical trials testing the nootropic effects of G. biloba extracts in healthy people. Hum Psychopharmacol 22:265–278
Biegler KA, Chaoul MA, Cohen L (2009) Cancer, cognitive impairment, and meditation. Acta Oncol 48:18–26
Xiong GL, Doraiswamy PM (2009) Does meditation enhance cognition and brain plasticity? Ann N Y Acad Sci 1172:63–69
Pagnoni G, Cekic M (2007) Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging 28:1623–1627
Zylowska L, Ackerman DL, Yang MH, Futrell JL, Horton NL, Hale TS, Pataki C, Smalley SL (2008) Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord 11:737–746
Schoenberger NE, Shif SC, Esty ML, Ochs L, Matheis RJ (2001) Flexyx Neurotherapy System in the treatment of traumatic brain injury: an initial evaluation. J Head Trauma Rehabil 16:260–274
van den Berg M, Visser A, Schoolmeesters A, Edelman P, van den Borne B (2006) Evaluation of haptotherapy for patients with cancer treated with chemotherapy at a day clinic. Patient Educ Couns 60:336–343
van der Watt G, Laugharne J, Janca A (2008) Complementary and alternative medicine in the treatment of anxiety and depression. Curr Opin Psychiatry 21:37–42
Mamtani R, Cimino A (2002) A primer of complementary and alternative medicine and its relevance in the treatment of mental health problems. Psychiatr Q 73:367–381
Pilkington K (2010) Anxiety, depression and acupuncture: a review of the clinical research. Auton Neurosci 157:91–95
Pilkington K, Kirkwood G, Rampes H, Cummings M, Richardson J (2007) Acupuncture for anxiety and anxiety disorders–a systematic literature review. Acupunct Med 25:1–10
Spiegel D, Cardena E (1990) New uses of hypnosis in the treatment of posttraumatic stress disorder. J Clin Psychiatry 51(Suppl):39–46
Schnur JB, Kafer I, Marcus C, Montgomery GH (2008) Hypnosis to manage distress related to medical procedures: a meta-analysis. Contemp Hypn 25:114–128
Butler LD, Symons BK, Henderson SL, Shortliffe LD, Spiegel D (2005) Hypnosis reduces distress and duration of an invasive medical procedure for children. Pediatrics 115:e77–e85
Schnur JB, Bovbjerg DH, David D, Tatrow K, Goldfarb AB, Silverstein JH, Weltz CR, Montgomery GH (2008) Hypnosis decreases presurgical distress in excisional breast biopsy patients. Anesth Analg 106:440–444
Blankfield RP (1991) Suggestion, relaxation, and hypnosis as adjuncts in the care of surgery patients: a review of the literature. Am J Clin Hypn 33:172–186
Luebbert K, Dahme B, Hasenbring M (2001) The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology 10:490–502
Janbozorgi M, Zahirodin A, Norri N, Ghafarsamar R, Shams J (2009) Providing emotional stability through relaxation training. East Mediterr Health J 15:629–638
Siev J, Chambless DL (2007) Specificity of treatment effects: cognitive therapy and relaxation for generalized anxiety and panic disorders. J Consult Clin Psychol 75:513–522
Manzoni GM, Pagnini F, Gorini A, Preziosa A, Castelnuovo G, Molinari E, Riva G (2009) Can relaxation training reduce emotional eating in women with obesity? An exploratory study with 3 months of follow-up. J Am Diet Assoc 109:1427–1432
Lundgren J, Carlsson SG, Berggren U (2006) Relaxation versus cognitive therapies for dental fear–a psychophysiological approach. Health Psychol 25:267–273
Peebles KA, Baker RK, Kurz EU, Schneider BJ, Kroll DJ (2001) Catalytic inhibition of human DNA topoisomerase IIalpha by hypericin, a naphthodianthrone from St. John’s wort (Hypericum perforatum). Biochem Pharmacol 62:1059–1070
He SM, Yang AK, Li XT, Du YM, Zhou SF (2010) Effects of herbal products on the metabolism and transport of anticancer agents. Expert Opin Drug Metab Toxicol 6:1195–1213
Norton SA (1998) Herbal medicines in Hawaii from tradition to convention. Hawaii Med J 57:382–386
Stevinson C, Huntley A, Ernst E (2002) A systematic review of the safety of kava extract in the treatment of anxiety. Drug Saf 25:251–261
Moquin B, Blackman MR, Mitty E, Flores S (2009) Complementary and alternative medicine (CAM). Geriatr Nurs 30:196–203
Corner J, Yardley J, Maher EJ, Roffe L, Young T, Maslin-Prothero S, Gwilliam C, Haviland J, Lewith G (2009) Patterns of complementary and alternative medicine use among patients undergoing cancer treatment. Eur J Cancer Care (Engl) 18:271–279
Wells RE, Phillips RS, Schachter SC, McCarthy EP (2010) Complementary and alternative medicine use among US adults with common neurological conditions. J Neurol 257:1822–1831
Shakeel M, Newton JR, Ah-See KW (2009) Complementary and alternative medicine use among patients undergoing otolaryngologic surgery. J Otolaryngol Head Neck Surg 38:355–561
Shakeel M, Trinidade A, Ah-See KW (2010) Complementary and alternative medicine use by otolaryngology patients: a paradigm for practitioners in all surgical specialties. Eur Arch Otorhinolaryngol 267:961–971
Shelley BM, Sussman AL, Williams RL, Segal AR, Crabtree BF, Rios Net Clinicians (2009) ‘They don’t ask me so I don’t tell them’: patient–clinician communication about traditional, complementary, and alternative medicine. Ann Fam Med 7:139–147
Acknowledgments
The authors thank Prof. Aaron Polliack for his contribution to the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avisar, A., River, Y., Schiff, E. et al. Chemotherapy-related cognitive impairment: does integrating complementary medicine have something to add? Review of the literature. Breast Cancer Res Treat 136, 1–7 (2012). https://doi.org/10.1007/s10549-012-2211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2211-5