Abstract
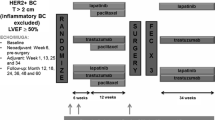
Monitoring of left ventricular ejection fraction (LVEF) is the current standard for detection of trastuzumab-induced cardiotoxicity; however, time-to-diagnosis and cost of assessment are suboptimal in women with early-stage breast cancer. We assessed the utility of B-type natriuretic peptide (BNP), high-sensitivity C-reactive protein (hs-CRP), and cardiac troponin I (cTnI) as serum biomarkers for early detection of trastuzumab-induced cardiotoxicity. Fifty-four women with human epidermal growth factor receptor 2 (HER2)-positive early-stage breast cancer were prospectively enrolled, and the relationship between elevated serum BNP, hs-CRP, and cTnI levels and clinically significant decreases in LVEF was examined. LVEF was monitored at 3–4 month intervals during trastuzumab treatment. Laboratory testing for candidate biomarkers was repeated every 3 weeks with each cycle of trastuzumab. Trastuzumab-induced cardiotoxicity was defined as a decrease in LVEF of ≥15 % or to a value below 50 %. A clinically significant decrease in LVEF was observed in 28.6 % of women. Abnormal hs-CRP (≥3 mg/L) predicted decreased LVEF with a sensitivity of 92.9 % (95 % CI 66.1–99.8) and specificity of 45.7 % (95 % CI 28.8–63.4), and subjects with normal hs-CRP levels (<3 mg/L) have 94.1 % negative predictive 94.1 % (95 % CI 70.3–99.9) suggesting that normal hs-CRP levels may be associated with low future risk for decreased LVEF; however, no association with BNP or cTnI was observed. A false positive would have a relatively low associated cost in breast cancer patients undergoing adjuvant trastuzumab therapy and would indicate continuation of routine observation during treatment through traditional means. The maximum hs-CRP value was observed a median of 78 days prior to detection of cardiotoxicity by decreased LVEF, and those with normal levels were at lower risk for cardiotoxicity. Regular monitoring of hs-CRP holds promise as a biomarker for identifying women with early-stage breast cancer at low risk for asymptomatic trastuzumab-induced cardiotoxicity. To our knowledge, this is the first study documenting the utility of a less expensive, reproducible, easily obtainable biomarker with rapid results for evaluating cardiotoxicity related to trastuzumab therapy.



Similar content being viewed by others
References
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Costa RB, Kurra G, Greenberg L, Geyer CE (2010) Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol 21:2153–2160
Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV (2007) Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 7:153
Chien AJ, Rugo HS (2010) The cardiac safety of trastuzumab in the treatment of breast cancer. Expert Opin Drug Saf 9:335–346
Routledge HC, Rea DW, Steeds RP (2006) Monitoring the introduction of new drugs—Herceptin to cardiotoxicity. Clin Med 6:478–481
Tripathy D, Slamon DJ, Cobleigh M, Arnold A, Saleh M, Mortimer JE, Murphy M, Stewart SJ (2004) Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 22:1063–1070
Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23:7820–7826
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215–1221
Kazanegra R, Cheng V, Garcia A, Krishnaswamy P, Gardetto N, Clopton P, Maisel A (2001) A rapid test for B-type natriuretuc peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail 7:21–29
Krishnaswamy P, Lubien E, Clopton P, Koon J, Kazanegra R, Wanner R, Gardetto N, Garcia A, DeMaria A, Maisel AS (2001) Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med 111:274–279
Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, Cinieri S, Martinelli G, Fiorentini C, Cipolla CM (2002) Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol 13:710–715
Lubien E, DeMaria A, Krishnaswamy P, Copton P, Koon J, Kazanegra R, Gardetto N, Wanner E, Maisel AS (2002) Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 105:595–601
Sandri MT, Cardinale D, Zorzino L, Passerini R, Lentati P, Martinoni A, Martinenlli G, Cipolla CM (2003) Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin Chem 49:248–252
Xue C, Feng Y, Wo J, Li Y (2006) Prognostic value of high-sensitivity C-reactive protein in patients with chronic heart failure. N Z Med J 119:U2314
Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D (2007) Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 12:265–274
Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, Jasper S, Klein AL (2008) Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol 101:370–373
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention; American Heart Association (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511
Heeschen C, Hamm CW, Goldmann B, Deu A, Langenbrink L, White HD (1999) Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. PRISM Study Investigators. Platelet Receptor Inhibition in Ischemic Syndrome Management. Lancet 354:1757–1762
Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23:7811–7819
Chapman JA, Meng D, Shepherd L, Parulekar W, Ingle JN, Muss HB, Palmer M, Yu C, Goss PE (2008) Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst 100:252–260
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843
Arruda-Olson AM, Enriquez-Sarano M, Bursi F, Weston SA, Jaffe AS, Killian JM, Roger VL (2010) Left ventricular function and C-reactive protein levels in acute myocardial infarction. Am J Cardiol 105:917–921
Arroyo-Espiliguero R, Avanzas P, Quiles J, Kaski JC (2009) Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis 204:239–243
Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG (2007) Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J 153:1048–1055
Tsuruda T, Kato J, Sumi T, Mishima K, Masuyama H, Nakao H, Imamura T, Eto T, Kitamura K (2007) Combined use of brain natriuretic peptide and C-reactive protein for predicting cardiovascular risk in outpatients with type 2 diabetes mellitus. Vasc Health Risk Manag 3:417–423
Ndrepepa G, Kastrati A, Braun S, Mehilli J, Niemöller K, von Beckerath N, von Beckerath O, Vogt W, Schömig A (2006) N-terminal probrain natriuretic peptide and C-reactive protein in stable coronary heart disease. Am J Med 119(355):e1–e8
Ishikawa C, Tsutamoto T, Fujii M, Sakai H, Tanaka T, Horie M (2006) Prediction of mortality by high-sensitivity C-reactive protein and brain natriuretic peptide in patients with dilated cardiomyopathy. Circ J 70:857–863
Kutteh LA, Hobday T, Jaffe A, LaPlant B, Hillman D, Kaufman P, Davidson N, Martino S, Moreno A, Perez E, Central Cancer Treatment Group (2007) A correlative study of cardiac biomarkers and left ventricular ejection fraction (LVEF) from N9831, a phase III randomized trial of chemotherapy and trastuzumab as adjuvant therapy for HER2-positive breast cancer. J Clin Oncol 25(18):579
Goel S, Simes RJ, Beith JM (2011) Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia Pac J Clin Oncol 7:276–280
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS (2011) The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with trastuzumab therapy. J Am Coll Cardiol 57:2263–2270
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 107:1375–1380
Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nolè F, Veglia F, Cipolla CM (2010) Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 28:3910–3916
Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT (2011) Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res 17:3490–3499
Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE (1999) Prevalence of heart failure and left ventricular dysfunction in the general population: The Rotterdam Study. Eur Heart J 20:447–455
Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D (1999) Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33:1948–1955
Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA (2002) Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 137:631–639
Acknowledgments
The authors thank Marie Fleisner of the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication for proofreading/copyediting assistance. Funding for this study was provided by the Marshfield Clinic Physician Research Funds and the Marshfield Clinic Research Foundation Disease Specific Funds.
Conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onitilo, A.A., Engel, J.M., Stankowski, R.V. et al. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat 134, 291–298 (2012). https://doi.org/10.1007/s10549-012-2039-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2039-z