Abstract
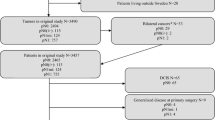
The early spread of tumor cells in primary breast cancer patients may occur either through lymphatic or hematogenous dissemination. Lymph node (LN) status and presence of disseminated tumor cells (DTC) in bone marrow (BM) are independent predictors of poor outcome. It is unknown which factors determine one or the other route of tumor cell spread and whether lymphatic and hematogenous tumor cell dissemination are two independent processes. This study is aimed to compare the DTC status in clinically node-negative (N0) breast cancer patients with their sentinel LN status and to investigate predictors of BM and LN involvement. The DTC status of 1,345 clinically N0 breast cancer patients who underwent SLN biopsy during initial surgery was investigated. BM and LN status were compared and predictors of hematogenous and lymphatic tumor cell spread were investigated. DTCs were present in the BM of 181 (13%) patients. LN involvement was found in 348 (26%) patients. There was no correlation between LN and BM status: 137 of 997 nodal-negative patients (14%) had BM involvement and 44 of 348 nodal-positive patients (13%) were positive for DTCs (P = 0.649). The presence of DTCs was not influenced by tumorbiological factors. Conversely, a high correlation between nodal status and tumor size, histology, ER-status and lymph vessel invasion was found. Hematogenous and lymphatic tumor spread seem to be because of independent pathways of cancer progression.


Similar content being viewed by others
Abbreviations
- BM:
-
Bone marrow
- CK:
-
Cytokeratin
- DTC:
-
Disseminated tumor cell(s)
- LN:
-
Lymph node
- SLN:
-
Sentinel lymph node
References
Klein CA (2009) Parallel progression of primary tumours and metastases. Nat Rev Cancer 9(4):302–312. doi:10.1038/nrc2627
Leone BA, Romero A, Rabinovich MG, Vallejo CT, Bianco A, Perez JE, Machiavelli M, Rodriguez R, Alvarez LA (1988) Stage IV breast cancer: clinical course and survival of patients with osseous versus extraosseous metastases at initial diagnosis. The GOCS (Grupo Oncologico Cooperativo del Sur) experience. Am J Clin Oncol 11(6):618–622
Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmuller G, Schlimok G (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342(8):525–533
Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Qvist H, Schlichting E, Sauer T, Janbu J, Harbitz T, Naume B (2003) Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol 21(18):3469–3478
Gerber B, Krause A, Muller H, Richter D, Reimer T, Makovitzky J, Herrnring C, Jeschke U, Kundt G, Friese K (2001) Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol 19(4):960–971
Pierga JY, Bonneton C, Vincent-Salomon A, de Cremoux P, Nos C, Blin N, Pouillart P, Thiery JP, Magdelenat H (2004) Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res 10(4):1392–1400
Gebauer G, Fehm T, Merkle E, Beck EP, Lang N, Jager W (2001) Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol 19(16):3669–3674
Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC (1999) Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet 354(9174):197–202
Diel IJ, Kaufmann M, Costa SD, Holle R, von Minckwitz G, Solomayer EF, Kaul S, Bastert G (1996) Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst 88(22):1652–1658
Fehm T, Becker S, Pergola-Becker G, Kramer B, Gruber I, Sotlar K, Kurek R, Wallwiener D, Solomayer E (2004) Influence of tumor biological factors on tumor cell dissemination in primary breast cancer. Anticancer Res 24(6):4211–4216
Bauer KD, de la Torre-Bueno J, Diel IJ, Hawes D, Decker WJ, Priddy C, Bossy B, Ludmann S, Yamamoto K, Masih AS, Espinoza FP, Harrington DS (2000) Reliable and sensitive analysis of occult bone marrow metastases using automated cellular imaging. Clin Cancer Res 6(9):3552–3559
Borgen E, Naume B, Nesland JM, Kvalheim G, Beiske K, Fodstad O, Diel I, Solomayer EF, Theocharous P, Coombes RC, Smith BM, Wunder E, Marolleau JP, Garcia J, Pantel K (1999) Standardization of the immunocytochemical detection of cancer cells in BM and blood: I establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy 1(5):377–388. doi:10.1080/0032472031000141283
Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, Schindlbeck C, Wallwiener D, Borgen E, Naume B, Pantel K, Solomayer E (2006) A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 107(5):885–892
Molina R, Jo J, Filella X, Zanon G, Pahisa J, Mu noz M, Farrus B, Latre ML, Escriche C, Estape J, Ballesta AM (1998) c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: prognostic value. Breast Cancer Res Treat 51(2):109–119
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802
Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N, Shivers S, Berman C, Wells K, Rapaport D, Shons A, Horton J, Greenberg H, Nicosia S, Clark R, Cantor A, Reintgen DS (1996) Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 276(22):1818–1822
Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, Costa A, de Cicco C, Geraghty JG, Luini A, Sacchini V, Veronesi P (1997) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349(9069):1864–1867. doi:10.1016/S0140-6736(97)01004-0
Turner RR, Ollila DW, Krasne DL, Giuliano AE (1997) Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 226(3):271–276 Discussion: 276–278
Barnwell JM, Arredondo MA, Kollmorgen D, Gibbs JF, Lamonica D, Carson W, Zhang P, Winston J, Edge SB (1998) Sentinel node biopsy in breast cancer. Ann Surg Oncol 5(2):126–130
Naume B, Borgen E, Kvalheim G, Karesen R, Qvist H, Sauer T, Kumar T, Nesland JM (2001) Detection of isolated tumor cells in bone marrow in early-stage breast carcinoma patients: comparison with preoperative clinical parameters and primary tumor characteristics. Clin Cancer Res 7(12):4122–4129
Braun S, Cevatli BS, Assemi C, Janni W, Kentenich CR, Schindlbeck C, Rjosk D, Hepp F (2001) Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol 19(5):1468–1475
Aslakson CJ, Miller FR (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 52(6):1399–1405
Alitalo K, Carmeliet P (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1(3):219–227
Wong SY, Hynes RO (2006) Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 5(8):812–817
Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA (2003) From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA 100(13):7737–7742
Engel J, Eckel R, Kerr J, Schmidt M, Furstenberger G, Richter R, Sauer H, Senn HJ, Holzel D (2003) The process of metastasisation for breast cancer. Eur J Cancer 39(12):1794–1806
Engel J, Lebeau A, Sauer H, Holzel D (2006) Are we wasting our time with the sentinel technique? Fifteen reasons to stop axilla dissection. Breast 15(3):452–455. doi:10.1016/j.breast.2005.05.009
Michaelson JS, Silverstein M, Wyatt J, Weber G, Moore R, Halpern E, Kopans DB, Hughes K (2002) Predicting the survival of patients with breast carcinoma using tumor size. Cancer 95(4):713–723. doi:10.1002/cncr.10742
Klein CA, Holzel D (2006) Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle 5(16):1788–1798
Foulkes WD, Grainge MJ, Rakha EA, Green AR, Ellis IO (2009) Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat 117(1):199–204. doi:10.1007/s10549-008-0102-6
Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD, Bajdik CD, Chia SK (2008) Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer 8(3):249–256. doi:10.3816/CBC.2008.n.028
Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, Easton D, Lakhani SR (2007) Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res 9(1):R4. doi:10.1186/bcr1636
de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, Nortier JW, Rutgers EJ, Seynaeve C, Menke-Pluymers MB, Bult P, Tjan-Heijnen VC (2009) Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med 361(7):653–663. doi:10.1056/NEJMoa0904832
Diel IJ, Jaschke A, Solomayer EF, Gollan C, Bastert G, Sohn C, Schuetz F (2008) Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol 19(12):2007–2011. doi:10.1093/annonc/mdn429
Rack B, Juckstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M, Rammel G, Zwingers T, Sommer H, Friese K, Janni W (2010) Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 30(5):1807–1813
Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, Kaufmann M, Bastert G (1998) Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 339(6):357–363. doi:10.1056/NEJM199808063390601
Acknowledgments
We thank Dr. Florian Marx for his excellent statistical assistance and revising the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andreas D. Hartkopf and Malgorzata Banys have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hartkopf, A.D., Banys, M., Krawczyk, N. et al. Bone marrow versus sentinel lymph node involvement in breast cancer: a comparison of early hematogenous and early lymphatic tumor spread. Breast Cancer Res Treat 131, 501–508 (2012). https://doi.org/10.1007/s10549-011-1802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1802-x