Abstract
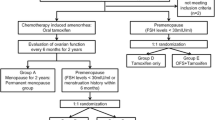
Ovarian ablation improves survival in premenopausal early breast cancer, but the potential added value by luteinizing hormone-releasing hormone (LHRH) agonists to tamoxifen is still not clear. The purpose of our study is to examine the efficacy of the LHRH agonist goserelin for adjuvant therapy of premenopausal breast cancer, the role of interaction between goserelin and tamoxifen and the impact of estrogen receptor (ER) content. A total of 927 patients were included in the Stockholm part of the Zoladex in Premenopausal Patients (ZIPP) trial. They were randomly allocated in a 2 × 2 factorial study design to goserelin, tamoxifen, the combination of goserelin and tamoxifen or no endocrine therapy for 2 years, with or without chemotherapy. This is formally not a preplanned subset analysis presenting the end point first event. In this Stockholm sub-study, at a median follow-up of 12.3 years, goserelin reduced the risk of first event by 32% (P = 0.005) in the absence of tamoxifen, and tamoxifen reduced the risk by 27% (P = 0.018) in the absence of goserelin. The combined goserelin and tamoxifen treatment reduced the risk by 24% (P = 0.021) compared with no endocrine treatment. In highly ER-positive tumours, there were 29% fewer events among goserelin treated (P = 0.044) and a trend towards greater risk reduction depending on the level of ER content. The greatest risk reduction from goserelin treatment was observed among those not receiving tamoxifen (HR: 0.52, P = 0.007). In conclusion, goserelin as well as tamoxifen reduces the risk of recurrence in endocrine responsive premenopausal breast cancer. Women with strongly ER-positive tumours may benefit more from goserelin treatment. The combination of goserelin and tamoxifen is not superior to either modality alone. With the limitations of a subset trial, these data have to be interpreted cautiously.




Similar content being viewed by others
References
Davidson NE, O’Neill AM, Vukov AM, Osborne CK, Martino S, White DR, Abeloff MD (2005) Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from int 0101 (e5188). J Clin Oncol 23:5973–5982
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R, Marth C, Investigators A-T (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360:679–691
Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, Sainsbury R, Group L-aiEBCO (2007) Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 369:1711–1723
[No authors listed] (1996) Ovarian ablation in early breast cancer: overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 348:1189–1196
Fisher B, Redmond C, Brown A, Wickerham DL, Wolmark N, Allegra J, Escher G, Lippman M, Savlov E, Wittliff J (1983) Influence of tumor estrogen and progesterone receptor levels on the response to tamoxifen and chemotherapy in primary breast cancer. J Clin Oncol 1:227–241
Rutqvist LE, Cedermark B, Glas U, Johansson H, Nordenskjöld B, Skoog L, Somell A, Theve T, Friberg S, Askergren J (1987) The Stockholm trial on adjuvant tamoxifen in early breast cancer. Correlation between estrogen receptor level and treatment effect. Breast Cancer Res Treat 10:255–266
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thürlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol 25:3846–3852
Khoshnoud MR, Löfdahl B, Fohlin H, Fornander T, Stål O, Skoog L, Bergh J, Nordenskjöld B (2011) Immunohistochemistry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res Treat 126:421–430
Baum M, Hackshaw A, Houghton J, Rutqvist, Fornander T, Nordenskjold B, Nicolucci A, Sainsbury R, Group ZIC (2006) Adjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer 42:895–904
Hackshaw A, Baum M, Fornander T, Nordenskjold B, Nicolucci A, Monson K, Forsyth S, Reczko K, Johansson U, Fohlin H, Valentini M, Sainsbury R (2009) Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer. J Natl Cancer Inst 101:341–349
Goel S, Sharma R, Hamilton A, Beith J (2009) Lhrh agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev, CD004562
Wrange O, Nordenskjöld B, Gustafsson JA (1978) Cytosol estradiol receptor in human mammary carcinoma: an assay based on isoelectric focusing in polyacrylamide gel. Anal Biochem 85:461–475
Burton K (1956) A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62:315–323
Bonetti M, Gelber RD (2000) A graphical method to assess treatment–covariate interactions using the cox model on subsets of the data. Stat Med 19:2595–2609
Royston P, Sauerbrei W (2004) A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med 23:2509–2525
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T, Group AT (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Bergh J, Jönsson PE, Lidbrink E, Trudeau M, Eiermann W, Brattström D, Lindemann J, Wiklund F, Henriksson R (2009) First results from FACT: an open-label, randomized phase III study investigating loading dose of fulvestrant combined with anastrozole versus anastrozole at first relapse in hormone receptor positive breast cancer. In: Proceedings of the 32nd annual San Antonio breast cancer symposium (SABCS). San Antonio, TX
EBCTCG (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Sverrisdottir A, Nystedt M, Johansson H, Fornander T (2009) Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat 117:561–567
Sverrisdóttir A, Fornander T, Jacobsson H, von Schoultz E, Rutqvist LE (2004) Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol 22:3694–3699
Berglund G, Nystedt M, Bolund C, Sjödén PO, Rutqvist LE (2001) Effect of endocrine treatment on sexuality in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol 19:2788–2796
Nystedt M, Berglund G, Bolund C, Fornander T, Rutqvist LE (2003) Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol 21:1836–1844
Albain KS, Barlow WE, Ravdin PM, Farrar WB, Burton GV, Ketchel SJ, Cobau CD, Levine EG, Ingle JN, Pritchard KI, Lichter AS, Schneider DJ, Abeloff MD, Henderson IC, Muss HB, Green SJ, Lew D, Livingston RB, Martino S, Osborne CK, America BCIoN (2009) Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:2055–2063
Acknowledgments
This trial was supported by the Swedish Cancer Society and King Gustav V Jubilee Fund. Jonas Bergh’s research group was supported as well by The Swedish Research Fund, Stockholm County Council Research Funds (ALF/FoU), The Stockholm Cancer Society, The Swedish Breast Cancer Association (BRO), Karolinska Instituet, Stockholm County Council Research Strategy Initiative and Märit and Hans Rausing’s Initiative Against Breast Cancer. We would like to thank all contributing physicians participating in the trial at Karolinska-, Söder-, Danderyd-, St. Görans-, Visby-, Sabbatsberg-, Södetälje- and Huddinge Hospitals, as well as all present and former members of the Stockholm Breast Cancer Group. Finally, we like to thank the nursing staff at the breast units at Karolinska-, Danderyd-, Huddinge- and Söder Hospitals, contributing to the logistics of the trial, coordinating patient visits and examinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sverrisdottir, A., Johansson, H., Johansson, U. et al. Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat 128, 755–763 (2011). https://doi.org/10.1007/s10549-011-1593-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1593-0