Abstract
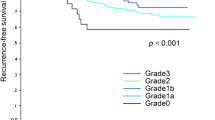
Neoadjuvant chemotherapy (NAC) is increasingly used for operable disease. However there are several pathological response classification systems and the correlation between the pathological response to NAC according to each system and the patient outcome is still under debate. From 1998 to 2006, 370 primary breast cancer patients underwent curative surgical treatment after NAC containing both anthracycline and taxane at the National Cancer Center Hospital. We retrospectively evaluated the clinical and pathological response using the cTMN, Fisher’s, Chevailler’s, and the Japanese Breast Cancer Society classification systems (JBCS) respectively, and analyzed the correlation between each pathological response and disease free survival (DFS). Ninety-five (26%) patients had tumor recurrence. The five-year DFS according to Fisher’s system was pCR, 80% and pINV, 63%. The five-year DFS according to Chevallier’s system was Grade 1, 83%, Grade 2, 85%, Grade 3, 62%, and Grade 4, 65%. The five-year DFS according to the JBSC system was Grade 3, 77%, Grade 2, 68%, Grade 1a, 68%, Grade 1b, 58%, and Grade 0, 52%. None of the pathological response systems reached a statistically significant difference. In the classification by the post-treatment number of metastatic axillary lymph nodes, the 5-year DFS was n = 0, 86%; n = 1–3, 64%; n = 4–9, 44%; and n > 10 positive: 25% (P < .0001). In pathologically node negative patients, there were no significant differences in the DFS among all the classification systems. All three classifications analyzed were considered inadequate as the prognostic marker of the long-term outcome after NAC and further studies are warranted to optimize the prediction.



Similar content being viewed by others
References
Fisher B, Bryant J, Wolmark N (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Bollet MA, Sigal-Zafrani B, Gambotti L et al (2006) Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: results of a phase II study. Eur J Cancer 42:2286–2295
Chevallier B, Roche H, Olivier JP et al (1993) Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 16:223–228
Japanese Breast Cancer Society (2005) General rules for clinical and pathological recording of breast cancer. Breast Cancer 12:S12–S14
Committee for production of histopathological criteria. Japanese breast cancer society (2001) Histopathological criteria for assessment of therapeutic response in breast cancer. Breast Cancer 8:1
Petit T, Wilt M, Velten M et al (2004) Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant Anthracycline-based chemotherapy. Eur J Cancer 40:205–211
Burcombe RJ, Makris A, Richman PI et al (2005) Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant Anthracycline chemotherapy for operable breast cancer. Br J Cancer 92:147–155
Estevez LG, Gradishar WJ (2004) Evidence-based use of neoadjuvant Taxane in operable and inoperable breast cancer. Clin Cancer Res 10:3249–3261
Jones RL, Lakhani SR, Ring AE (2006) Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer 94:358–362
Rajan R. Poniecka A, Smith TL et al (2004) Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer 100:1365–1373
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327
Bear HD, Anderson S, Smith RE et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24:1–9
Pierga JY, Mouret E, Laurence V et al (2003) Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer: the role of clinical response. Eur J Cancer 39:1089–1096
Amat S, Abrial C, Penault-Llorca F et al (2005) High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat 94:255–263
Chollet P, Amat S, Cure H et al (2002) Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 86:1041–1046
Hortobagyi GN, Buzdar AU, Kau SW (1998) Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 62:2507–2516
Cameron DA, Anderson ED, Levack P et al (1997) Primary systemic therapy for operable breast cancer-10-year survival data after chemotherapy and hormone therapy. Br J Cancer 76:1099–1105
Coudert BP, Arnold L, Moreau L et al (2006) Pre-operative systemic (neo-adjuvant) therapy with Trastuzumab and docetaxel for HER2-overexpressing stage II or stage III breast cancer: results of a multicenter phase II trial. Ann Oncol 17:409–419
Lenert JT, Vlastos G, Mirza NQ et al (1999) Primary tumor response to induction chemotherapy as a predictor of histological status of axillary nodes in operable breast cancer patients. An Surg Oncol 6:762–767
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shien, T., Shimizu, C., Seki, K. et al. Comparison among different classification systems regarding the pathological response of preoperative chemotherapy in relation to the long-term outcome. Breast Cancer Res Treat 113, 307–313 (2009). https://doi.org/10.1007/s10549-008-9935-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-9935-2