Abstract
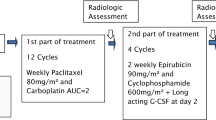
Purpose Granulocyte-colony stimulating factor (G-CSF) was used in CALGB 9741 to support dose-dense sequential chemotherapy with doxorubicin and cyclophosphamide (AC) followed by paclitaxel (P) (Citron et al. J Clin Oncol 21:1431–1439, 2003). However, myelosuppression is not known to be dose or schedule limiting for paclitaxel. We therefore conducted this trial to determine the need for routine G-CSF, using the pegylated product (pG-CSF), support during the paclitaxel component of dose-dense sequential chemotherapy in women with early stage breast carcinoma (BC). Patients and methods Eligible patients received dose-dense chemotherapy consisting of four cycles of AC followed by four cycles of P at two week intervals. pG-CSF (NeulastaTM) was administered after each of four cycles of AC but was held after P. Planned enrollment was 59 pts. Results Of the first 15 patients, nine completed therapy without delays due to neutropenia but 6 (40%) did not, leading to implementation of the pre-specified early termination rule. Overall, 85% of P doses were successfully delivered on time. The mean treatment delay for the entire group due to neutropenia was 0.75 days. There was no significant correlation between neutropenia and prior WBC, ANC, or concurrent treatment with trastuzumab. Pts with neutropenia tended to be younger (Mean age 43.5) and have a lower BSA (1.65 m2). There were no febrile episodes due to omission of pG-CSF. Conclusion When paclitaxel is administered in a dose-dense fashion without growth factor support brief treatment delays are common. Further study is needed to identify the minimal pG-CSF administration that will avoid treatment delays.
Similar content being viewed by others
References
Citron ML, Berry DA, Cirrincione C et al (2003) Trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 21:1431–1439
Akerley W, Herndon JE, Egorin MJ et al (2003) Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer 10:2480–2486
Kubista E, Holmes FA, Green MD et al (2003) Bone pain associated with once-per-cycle pegfilgrastim is similar to daily filgrastim in patients with breast cancer. Clin Breast Cancer 6:391–398
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-009-0482-2
Rights and permissions
About this article
Cite this article
Sugarman, S., Wasserheit, C., Hodgman, E. et al. A pilot study of dose-dense adjuvant paclitaxel without growth factor support for women with early breast carcinoma. Breast Cancer Res Treat 115, 609–612 (2009). https://doi.org/10.1007/s10549-008-0152-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0152-9