Summary
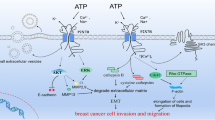
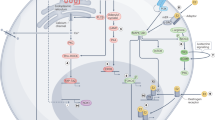
Prolonged exposure to 17β-estradiol (E2) is a key etiological factor for human breast cancer. The biological effects and carcinogenic effects of E2 are mediated via estrogen receptors (ERs), ERα and ERβ. Anti-estrogens, e.g. tamoxifen, and aromatase inhibitors have been used to treat ER-positive breast cancer. While anti-estrogen therapy is initially successful, a major problem is that most tumors develop resistance and the disease ultimately progresses, pointing to the need of developing alternative drugs targeting to other critical targets in breast cancer cells. We have identified that Na+, K+-ATPase, a plasma membrane ion pump, has unique/valuable properties that could be used as a potentially important target for breast cancer treatment: (a) it is a key player of cell adhesion and is involved in cancer progression; (b) it serves as a versatile signal transducer and is a target for a number of hormones including estrogens and (d) its aberrant expression and activity are implicated in the development and progression of breast cancer. There are several lines of evidence indicating that ouabain and related digitalis (the potent inhibitors of Na+, K+-ATPase) possess potent anti-breast cancer activity. While it is not clear how the suggested anti-cancer activity of these drugs work, several observations point to ouabain and digitalis as being potential ER antagonists. We critically reviewed many lines of evidence and postulated a novel concept that Na+, K+-ATPase in combination with ERs could be important targets of anti-breast cancer drugs. Modulators, e.g. ouabain and related digitalis could be useful to develop valuable anti-breast cancer drugs as both Na+, K+-ATPase inhibitors and ER antagonists.
Similar content being viewed by others
References
Pike MC, Spicer DV, Dahmoush L, Press MF, Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk Epidemiol Rev15:17–35, 1993
Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH, Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells J Steroid Biochem Mol Biol 87:1–25, 2003
Russo J, Lareef MH, Tahin Q, Hu YF, Slater C, Ao X, Russo IH, 17Beta-estradiol is carcinogenic in human breast epithelial cells J Steroid Biochem Mol Biol 80:149–162, 2002
Kelsey JL, Gammon MD, John EM, Reproductive factors and breast cancer Epidemiol Rev 15:36–47, 1993
Bernstein L, Ross RK, Endogenous hormones and breast cancer risk Epidemiol Rev 15:48–65, 1993
Feigelson HS, Henderson BE, Estrogens and breast cancer Carcinogenesis 17:2279–2284, 1996
Tsai MJ, O’Malley BW, Molecular mechanisms of action of steroid/thyroid receptor superfamily members Annu Rev Biochem 63:451–486, 1994
Pettersson K, Gustafsson JA, Role of estrogen receptor beta in estrogen action Annu Rev Physiol 63:165–192, 2001
Pettersson K, Delaunay F, Gustafsson JA, Estrogen receptor beta acts as a dominant regulator of estrogen signaling Oncogene 19:4970–4978, 2000
Beato M, Truss M, Chavez S, Control of transcription by steroid hormones Ann N Y Acad Sci 784:93–123, 1996
Beato M, Sanchez-Pacheco A, Interaction of steroid hormone receptors with the transcription initiation complex Endocr Rev 17:587–609, 1996
Evans RM, The steroid and thyroid hormone receptor superfamily Science, 240:889–895, 1988
Swerdlow AJ, Jones ME, Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case–control study J Natl Cancer Inst 97:375–384, 2005
Chung CT, Carlson RW, Adjuvant aromatase inhibitors following tamoxifen for early-stage breast cancer in postmenopausal women: what do we really know? Clin Breast Cancer 5(Suppl 1):S18–S23, 2004
Jensen EV, Jordan VC, The estrogen receptor: a model for molecular medicine Clin Cancer Res 9:1980–1989, 2003
Muss HB, Endocrine therapy for advanced breast cancer: a review Breast Cancer Res Treat 21:15–26, 1992
Jones KL, Buzdar AU, A review of adjuvant hormonal therapy in breast cancer Endocr Relat Cancer 11:391–406, 2004
Michaud LB, Adjuvant use of aromatase inhibitors in postmenopausal women with breast cancer Am J Health Syst Pharm 62:266–273, 2005
Mouridsen HT, Aromatase inhibitors in advanced breast cancer Semin Oncol 31:3–8, 2004
Viale PH, Aromatase inhibitor agents in breast cancer: evolving practices in hormonal therapy treatment Oncol Nurs Forum 32:343–353, 2005
Howell A, Dowsett M, Endocrinology and hormone therapy in breast cancer: aromatase inhibitors versus antioestrogens Breast Cancer Res 6:269–274, 2004
Yue W, Wang JP, Hamilton CJ, Demers LM, Santen RJ, In situ aromatization enhances breast tumor estradiol levels and cellular proliferation Cancer Res 58:927–932, 1998
Santen RJ, Martel J, Hoagland M, Naftolin F, Roa L, Harada N, Hafer L, Zaino R, Pauley R, Santner S, Demonstration of aromatase activity and its regulation in breast tumor and benign breast fibroblasts Breast Cancer Res Treat 49 Suppl 1:S93–S99; 1998. discussion S109–S119,
Santen RJ, To block estrogen’s synthesis or action: that is the question J Clin Endocrinol Metab 87:3007–3012, 2002
Chen S, Zhou D, Okubo T, Kao YC, Yang C, Breast tumor aromatase: functional role and transcriptional regulation Endocr Relat Cancer 6:149–156, 1999
Chen S, Itoh T, Wu K, Zhou D, Yang C, Transcriptional regulation of aromatase expression in human breast tissue J Steroid Biochem Mol Biol 83:93–99, 2002
de Jong PC, Blankenstein MA, van de Ven J, Nortier JW, Blijham GH, Thijssen JH, Importance of local aromatase activity in hormone-dependent breast cancer: a review Breast 10:91–99, 2001
Ackermann U, Geering K, Mutual dependence of Na, K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport FEBS Lett 269:105–108, 1990
Geering K, Subunit assembly and functional maturation of Na, K-ATPase J Membr Biol 115:109–121, 1990
Geering K, Theulaz I, Verrey F, Hauptle MT, Rossier BC, A role for the beta-subunit in the expression of functional Na+–K+–ATPase in Xenopus oocytes Am J Physiol 257:C851–C858, 1989
Fambrough DM, The sodium pump becomes a family Trends Neurosci 11:325–328, 1988
Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM, Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump J Biol Chem 263:4347–4354, 1988
Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M, The polarized expression of Na+, K+-ATPase in epithelia depends on the association between {beta}-subunits located in neighboring cells Mol Biol Cell 16:1071–1081, 2005
Horowitz B, Eakle KA, Scheiner-Bobis G, Randolph GR, Chen CY, Hitzeman RA, Farley RA, Synthesis and assembly of functional mammalian Na, K-ATPase in yeast J Biol Chem 265:4189–4192, 1990
Noguchi S, Mishina M, Kawamura M, Numa S, Expression of functional (Na+ + K+)-ATPase from cloned cDNAs FEBS Lett 225:27–32, 1987
Sweadner KJ, Wetzel RK, Arystarkhova E, Genomic organization of the human FXYD2 gene encoding the gamma subunit of the Na, K-ATPase Biochem Biophys Res Commun 279:196–201, 2000
Blanco G, Mercer RW, Isozymes of the Na–K-ATPase: heterogeneity in structure, diversity in function Am J Physiol 275:F633–F650, 1998
Cereijido M, Contreras RG, Shoshani L, Cell adhesion, polarity, and epithelia in the dawn of metazoans Physiol Rev 84:1229–1262, 2004
Yu SP, Na(+), K(+)-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death Biochem Pharmacol 66:1601–1609, 2003
Vogel T, Guo NH, Krutzsch HC, Blake DA, Hartman J, Mendelovitz S, Panet A, Roberts DD, Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin J Cell Biochem 53:74–84, 1993
Vogel T, Blake DA, Whikehart DR, Guo NH, Zabrenetzky VS, Roberts DD, Specific simple sugars promote chemotaxis and chemokinesis of corneal endothelial cells J Cell Physiol 157:359–366, 1993
Woo AL, James PF, Lingrel JB, Sperm motility is dependent on a unique isoform of the Na, K-ATPase J Biol Chem, 275:20693–20699, 2000
Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M, Relationship between Na(+), K(+)-ATPase and cell attachment J Cell Sci 112(Pt 23):4223–4232, 1999
Barwe SP, Anilkumar G, Moon SY, Zheng Y, Whitelegge JP, Rajasekaran SA, Rajasekaran AK, Novel role for Na, K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility Mol Biol Cell 16:1082–1094, 2005
Avila J, Alvarez de la Rosa D, Gonzalez-Martinez LM, Lecuona E, Martin-Vasallo P, Structure and expression of the human Na, K-ATPase beta 2-subunit gene Gene 208:221–227, 1998
Kometiani P, Liu L, Askari A, Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells Mol Pharmacol 67:929–936, 2005
Liguri G, Taddei N, Nassi P, Latorraca S, Nediani C, Sorbi S, Changes in Na+, K(+)-ATPase, Ca2(+)-ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer’s disease Neurosci Lett 112:338–342, 1990
Chauhan NB, Lee JM, Siegel GJ, Na, K-ATPase mRNA levels and plaque load in Alzheimer’s disease J Mol Neurosci 9:151–166, 1997
Chauhan N, Siegel G, Differential expression of Na, K-ATPase alpha-isoform mRNAs in aging rat cerebellum J Neurosci Res 47:287–299, 1997
Dickey CA, Gordon MN, Wilcock DM, Herber DL, Freeman MJ, Morgan D, Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice BMC Neurosci 6:7, 2005
Tsimarato M, Coste TC, Djemli-Shipkolye A, Daniel L, Shipkolye F, Vague P, Raccah D, Evidence of time-dependent changes in renal medullary Na, K-ATPase activity and expression in diabetic rats Cell Mol Biol (Noisy-le-grand) 47:239–245, 2001
Liu X, Songu-Mize E, Alterations in alpha subunit expression of cardiac Na+, K+-ATPase in spontaneously hypertensive rats: effect of antihypertensive therapy Eur J Pharmacol 327:151–156, 1997
Espineda C, Seligson DB, James Ball W, Jr., Rao J, Palotie A, Horvath S, Huang Y, Shi T, Rajasekaran AK, Analysis of the Na, K-ATPase alpha- and beta-subunit expression profiles of bladder cancer using tissue microarrays Cancer 97:1859–1868, 2003
Blok LJ, Chang GT, Steenbeek-Slotboom M, van Weerden WM, Swarts HG, De Pont JJ, van Steenbrugge GJ, Brinkmann AO, Regulation of expression of Na+, K+-ATPase in androgen-dependent and androgen-independent prostate cancer Br J Cancer 81:28–36, 1999
Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, Rajasekaran AK, Na, K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells Am J Physiol Cell Physiol 284:C1497–C1507, 2003
Rajasekaran SA, Gopal J, Rajasekaran AK, Expression of Na, K-ATPase beta-subunit in transformed MDCK cells increases the translation of the Na, K-ATPase alpha-subunit Ann NY Acad Sci 986:652–654, 2003
Rajasekaran SA, Ball WJ, Jr., Bander NH, Liu H, Pardee JD, Rajasekaran AK, Reduced expression of beta-subunit of Na, K-ATPase in human clear-cell renal cell carcinoma J Urol 162:574–580, 1999
Davies RJ, Sandle GI, Thompson SM, Inhibition of the Na+, K(+)-ATPase pump during induction of experimental colon cancer Cancer Biochem Biophys 12:81–94, 1991
Lee S, Baek M, Yang H, Bang YJ, Kim WH, Ha JH, Kim DK, Jeoung DI, Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays Cancer Lett 184:197–206, 2002
Espineda CE, Chang JH, Twiss J, Rajasekaran SA, Rajasekaran AK, Repression of Na, K-ATPase beta1-subunit by the transcription factor snail in carcinoma Mol Biol Cell 15:1364–1373, 2004
Rajasekaran AK, Gopal J, Rajasekaran SA, Na, K-ATPase in the regulation of epithelial cell structure Ann NY Acad Sci 986:649–651, 2003
Rajasekaran AK, Rajasekaran SA, Role of Na–K-ATPase in the assembly of tight junctions Am J Physiol Renal Physiol 285:F388–F396, 2003
Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S, Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast Oncogene 22:2021–2033, 2003
Morita K, Tsukita S, Miyachi Y, Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen’s disease Br J Dermatol 151:328–334, 2004
Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W, Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta–catenin complex in cells transformed with a temperature-sensitive v-SRC gene J Cell Biol 120:757–766, 1993
Cereijido M, Shoshani L, Contreras RG, Molecular physiology and pathophysiology of tight junctions. I. Biogenesis of tight junctions and epithelial polarity Am J Physiol Gastrointest Liver Physiol 279:G477–G482, 2000
Cereijido M, Valdes J, Shoshani L, Contreras RG, Role of tight junctions in establishing and maintaining cell polarity Annu Rev Physiol 60:161–177, 1998
Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD, Polarized monolayers formed by epithelial cells on a permeable and translucent support J Cell Biol 77:853–880, 1978
Cereijido M, Contreras RG, Gonzalez-Mariscal L, Development and alteration of polarity Annu Rev Physiol 51:785–795, 1989
Itoh M, Bissell MJ, The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis J Mammary Gland Biol Neoplasia 8:449–462, 2003
Okegawa T, Pong RC, Li Y, Hsieh JT, The role of cell adhesion molecule in cancer progression and its application in cancer therapy Acta Biochim Pol 51:445–457, 2004
Wheelock MJ, Johnson KR, Cadherin-mediated cellular signaling Curr Opin Cell Biol 15:509–514, 2003
Wheelock MJ, Johnson KR, Cadherins as modulators of cellular phenotype Annu Rev Cell Dev Biol 19:207–235, 2003
Takeichi M, Cadherin cell adhesion receptors as a morphogenetic regulator Science 251:1451–1455, 1991
Gumbiner BM, Regulation of cadherin adhesive activity J Cell Biol 148:399–404, 2000
Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, Larre I, Cereijido M, Ouabain binding to Na+, K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus J Membr Biol 198:147–158, 2004
Balda MS, Garrett MD, Matter K, The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density J Cell Biol 160:423–432, 2003
Gonzalez-Mariscal L, Chavez de Ramirez B, Lazaro A, Cereijido M, Establishment of tight junctions between cells from different animal species and different sealing capacities J Membr Biol 107:43–56, 1989
Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Monroy AO, Roldan ML, Fiorentino R, Cereijido M, E-Cadherin and tight junctions between epithelial cells of different animal species Pflugers Arch 444:467–475, 2002
Mullin JM, Epithelial barriers, compartmentation, and cancer Sci STKE: 2004 pe2, 2004
Contreras RG, Lazaro A, Bolivar JJ, Flores-Maldonado C, Sanchez SH, Gonzalez-Mariscal L, Garcia-Villegas MR, Valdes J, Cereijido M, A novel type of cell–cell cooperation between epithelial cells J Membr Biol 145:305–310, 1995
Contreras RG, Lazaro A, Mujica A, Gonzalez-Mariscal L, Valdes J, Garcia-Villegas MR, Cereijido M, Ouabain resistance of the epithelial cell line (Ma104) is not due to lack of affinity of its pumps for the drug J Membr Biol 145:295–300, 1995
Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr., Bander NH, Peralta Soler A, Rajasekaran AK, Na, K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility Mol Biol Cell 12:279–295, 2001
Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK, Na, K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells Mol Biol Cell 12:3717–3732, 2001
Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M, Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin J Membr Biol 122:193–202, 1991
Contreras RG, Avila G, Gutierrez C, Bolivar JJ, Gonzalez-Mariscal L, Darzon A, Beaty G, Rodriguez-Boulan E, Cereijido M, Repolarization of Na+–K+ pumps during establishment of epithelial monolayers Am J Physiol 257:C896–C905, 1989
Martin TA, Watkins G, Mansel RE, Jiang WG, Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer Eur J Cancer 40:2717–2725, 2004
Hoover KB, Liao SY, Bryant PJ, Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity Am J Pathol 153:1767–1773, 1998
Mauro L, Bartucci M, Morelli C, Ando S, Surmacz E, IGF-I receptor-induced cell–cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1 J Biol Chem 276:39892–39897, 2001
Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, Korc M, Buchler MW, Friess H, Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer Pancreas 23:259–265, 2001
Chlenski A, Ketels KV, Engeriser JL, Talamonti MS, Tsao MS, Koutnikova H, Oyasu R, Scarpelli DG, zo-2 gene alternative promoters in normal and neoplastic human pancreatic duct cells Int J Cancer 83:349–358, 1999
Chlenski A, Ketels KV, Tsao MS, Talamonti MS, Anderson MR, Oyasu R, Scarpelli DG, Tight junction protein ZO-2 is differentially expressed in normal pancreatic ducts compared to human pancreatic adenocarcinoma Int J Cancer 82:137–144, 1999
Tokunaga Y, Tobioka H, Isomura H, Kokai Y, Sawada N, Expression of occludin in human rectal carcinoid tumours as a possible marker for glandular differentiation Histopathology 44:247–250, 2004
Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N, Occludin expression decreases with the progression of human endometrial carcinoma Hum Pathol 35:159–164, 2004
Tsukita S, Furuse M, Itoh M, Multifunctional strands in tight junctions Nat Rev Mol Cell Biol 2:285–293, 2001
Rangel LB, Agarwal R, D’Souza T, Pizer ES, Alo PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR, Morin PJ, Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas Clin Cancer Res 9:2567–2575, 2003
Hoevel T, Macek R, Mundigl O, Swisshelm K, Kubbies M, Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells J Cell Physiol 191:60–68, 2002
Soini Y, Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma Hum Pathol 35:1531–1536, 2004
Michl P, Buchholz M, Rolke M, Kunsch S, Lohr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM, Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin Gastroenterology 121:678–684, 2001
Al Moustafa AE, Alaoui-Jamali MA, Batist G, Hernandez-Perez M, Serruya C, Alpert L, Black MJ, Sladek R, Foulkes WD, Identification of genes associated with head and neck carcinogenesis by cDNA microarray comparison between matched primary normal epithelial and squamous carcinoma cells Oncogene 21:2634–2640, 2002
Sobel G, Paska C, Szabo I, Kiss A, Kadar A, Schaff Z, Increased expression of claudins in cervical squamous intraepithelial neoplasia and invasive carcinoma Hum Pathol 36:162–169, 2005
O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF, Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma Eur J Cancer 38:2252–2257, 2002
Bendardaf R, Lamlum H, Ristamaki R, Pyrhonen S, CD44 variant 6 expression predicts response to treatment in advanced colorectal cancer Oncol Rep 11:41–45, 2004
Gulmann C, Grace A, Leader M, Butler D, Patchett S, Kay E, CD44v6: a potential marker of malignant transformation in intestinal metaplasia of the stomach? An immunohistochemical study using tissue microarrays Eur J Gastroenterol Hepatol 15:981–986, 2003
Rys J, Kruczak A, Lackowska B, Jaszcz-Gruchala A, Brandys A, Stelmach A, Reinfuss M, The role of CD44v3 expression in female breast carcinomas Pol J Pathol 54:243–247, 2003
Peinado H, Portillo F, Cano A, Transcriptional regulation of cadherins during development and carcinogenesis Int J Dev Biol 48:365–375, 2004
Blaschuk OW, Munro SB, Farookhi R, E-cadherin, estrogens and cancer: is there a connection? Can J Oncol 4:291–301, 1994
MacCalman CD, Farookhi R, Blaschuk OW, Estradiol regulates E-cadherin mRNA levels in the surface epithelium of the mouse ovary Clin Exp Metastasis 12:276–282, 1994
MacCalman CD, Brodt P, Doublet JD, Jednak R, Elhilali MM, Bazinet M, Blaschuk OW, The loss of E-cadherin mRNA transcripts in rat prostatic tumors is accompanied by increased expression of mRNA transcripts encoding fibronectin and its receptor Clin Exp Metastasis 12:101–107, 1994
Byers SW, Sujarit S, Jegou B, Butz S, Hoschutzky H, Herrenknecht K, MacCalman C, Blaschuk OW, Cadherins and cadherin-associated molecules in the developing and maturing rat testis Endocrinology 134:630–639, 1994
Mandel LJ, Bacallao R, Zampighi G, Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions Nature 361:552–555, 1993
Piepenhagen PA, Nelson WJ, Differential expression of cell–cell and cell–substratum adhesion proteins along the kidney nephron Am J Physiol 269:C1433–C1449, 1995
Piepenhagen PA, Peters LL, Lux SE, Nelson WJ, Differential expression of Na(+)–K(+)-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron Am J Physiol 269:C1417–C1432, 1995
Rajasekaran SA, Gopal J, Espineda C, Ryazantsev S, Schneeberger EE, Rajasekaran AK, HPAF-II, a cell culture model to study pancreatic epithelial cell structure and function Pancreas 29:e77–e83, 2004
Graham C, Simmons NL, Functional organization of the bovine rumen epithelium Am J Physiol Regul Integr Comp Physiol 288:R173–R181, 2005
Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A, Cell signaling microdomain with Na, K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations J Biol Chem 278:50355–50361, 2003
Aizman O, Aperia A, Na, K-ATPase as a signal transducer Ann NY Acad Sci 986:489–496, 2003
Aperia A, Regulation of sodium/potassium ATPase activity: impact on salt balance and vascular contractility Curr Hypertens Rep 3:165–171, 2001
Alzamora R, Marusic ET, Gonzalez M, Michea L, Nongenomic effect of aldosterone on Na+, K+-adenosine triphosphatase in arterial vessels Endocrinology 144:1266–1272, 2003
Rodrigo R, Rivera G, Lucero Y, Larraguibel C, Effect of ethanol on regulation of (Na + K)-adenosine triphosphatase by aldosterone and dexamethasone in cultured renal papillary collecting duct cells Endocrine 19:301–304, 2002
Sharabani-Yosef O, Nir U, Sampson SR, Thyroid hormone up-regulates Na+/K+ pump alpha2 mRNA but not alpha2 protein isoform in cultured skeletal muscle Biochim Biophys Acta 1573:183–188, 2002
Banerjee B, Chaudhury S, Thyroidal regulation of different isoforms of NaKATPase in the primary cultures of neurons derived from fetal rat brain Life Sci 71:1643–1654, 2002
Chibalin AV, Kovalenko MV, Ryder JW, Feraille E, Wallberg-Henriksson H, Zierath JR, Insulin- and glucose-induced phosphorylation of the Na(+), K(+)-adenosine triphosphatase alpha-subunits in rat skeletal muscle Endocrinology 142:3474–3482, 2001
Bajpai M, Mandal SK, Chaudhury S, Identification of thyroid regulatory elements in the Na–K-ATPase alpha3 gene promoter Mol Biol Rep 28:1–7, 2001
Feraille E, Doucet A, Sodium–potassium–adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control Physiol Rev 81:345–418, 2001
Ewart HS, Klip A, Hormonal regulation of the Na(+)–K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity Am J Physiol 269:C295–C311, 1995
Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z, Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase J Biol Chem 279:17250–17259, 2004
Xie Z, Askari A, Na(+)/K(+)-ATPase as a signal transducer Eur J Biochem 269:2434–2439, 2002
Xie Z, Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer Cell Mol Biol (Noisy-le-grand) 47:383–390, 2001
Boelsterli UA, Rakhit G, Balazs T, Modulation by S-adenosyl-L-methionine of hepatic Na+, K+-ATPase, membrane fluidity, and bile flow in rats with ethinyl estradiol-induced cholestasis Hepatology 3:12–17, 1983
Berr F, Simon FR, Reichen J, Ethynylestradiol impairs bile salt uptake and Na–K pump function of rat hepatocytes Am J Physiol 247:G437–G443, 1984
Rosario J, Sutherland E, Zaccaro L, Simon FR, Ethinylestradiol administration selectively alters liver sinusoidal membrane lipid fluidity and protein composition Biochemistry 27:3939–3946, 1988
Reichen J, Paumgartner G, Relationship between bile flow and Na+, K+-adenosinetriphosphatase in liver plasma membranes enriched in bile canaliculi J Clin Invest 60:429–434, 1977
Melis MG, Troffa C, Manunta P, Pinna Parpaglia P, Soro A, Pala F, Madeddu P, Pazzola A, Tonolo G, Patteri G, et al, Effect of menstrual cycle hormones on cation transport in the red-cell membrane Boll Soc Ital Biol Sper 66:679–684, 1990
Ziegelhoffer A, Dzurba A, Vrbjar N, Styk J, Slezak J, Mechanism of action of estradiol on sodium pump in sarcolemma from the myocardium Bratisl Lek Listy 91:902–910, 1990
Kaur G, Sharma P, Bhardwaj S, GABA agonists and neurotransmitters metabolizing enzymes in steroid-primed OVX rats Mol Cell Biochem 167:107–111, 1997
Dzurba A, Ziegelhoffer A, Vrbjar N, Styk J, Slezak J, Estradiol modulates the sodium pump in the heart sarcolemma Mol Cell Biochem 176:113–118, 1997
Tsai ML, Lee CL, Tang MJ, Liu MY, Preferential reduction of Na+/K+ ATPase alpha3 by 17beta-estradiol influences contraction frequency in rat uteri Chin J Physiol 43:1–8, 2000
Tsai ML, Chang CC, Lee CL, Huang BY, The differential effects of tamoxifen and ICI 182, 780 on the reduction of Na+/K+ ATPase activity and spontaneous oscillations by 17beta-estradiol Chin J Physiol 46:55–62, 2003
Lee KH, Finnigan-Bunick C, Bahr J, Bunick D, Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta Biol Reprod 65:1534–1541, 2001
Hess RA, Bunick D, Bahr J, Oestrogen, its receptors and function in the male reproductive tract – a review Mol Cell Endocrinol 178:29–38, 2001
Aizman O, Uhlen P, Lal M, Brismar H, Aperia A, Ouabain, a steroid hormone that signals with slow calcium oscillations Proc Natl Acad Sci USA 98:13420–13424, 2001
Schoner W, Endogenous cardiotonic steroids Cell Mol Biol (Noisy-le-grand) 47:273–280, 2001
Kawamura A, Guo J, Itagaki Y, Bell C, Wang Y, Haupert GT, Jr., Magil S, Gallagher RT, Berova N, Nakanishi K, On the structure of endogenous ouabain Proc Natl Acad Sci USA 96:6654–6659, 1999
Doris PA, Bagrov AY, Endogenous sodium pump inhibitors and blood pressure regulation: an update on recent progress Proc Soc Exp Biol Med 218:156–167, 1998
Doris PA, Regulation of Na, K-ATPase by endogenous ouabain-like materials Proc Soc Exp Biol Med 205:202–212, 1994
Goto A, Yamada K, Ouabain-like factor Curr Opin Nephrol Hypertens 7:189–196, 1998
Stenkvist B, Pengtsson E, Dahlqvist B, Eriksson O, Jarkrans T, Nordin B, Cardiac glycosides and breast cancer, revisited N Engl J Med 306:484, 1982
Stenkvist B, Bengtsson E, Eriksson O, Holmquist J, Nordin B, Westman-Naeser S, Cardiac glycosides and breast cancer Lancet 1:563, 1979
Stenkvist B, Is digitalis a therapy for breast carcinoma? Oncol Rep 6:493–496, 1999
Stenkvist B, Bengtsson E, Eklund G, Eriksson O, Holmquist J, Nordin B, Westman-Naeser S, Evidence of a modifying influence of heart glucosides on the development of breast cancer Anal Quant Cytol 2:49–54, 1980
Repke KR, Schon R, Megges R, Weiland J, Nissen E, Matthes E, Potential suitability of Na+/K(+)-transporting ATPase in pre-screens for anti-cancer agents Anticancer Drug Des 10:177–187, 1995
Haux J, Klepp O, Spigset O, Tretli S, Digitoxin medication and cancer; case control and internal dose–response studies BMC Cancer 1:11, 2001
Haux J, Digitoxin is a potential anticancer agent for several types of cancer Med Hypotheses 53:543–548, 1999
Huang YT, Chueh SC, Teng CM, Guh JH, Investigation of ouabain-induced anticancer effect in human androgen-independent prostate cancer PC-3 cells Biochem Pharmacol 67:727–733, 2004
Yeh JY, Huang WJ, Kan SF, Wang PS, Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells J Urol 166:1937–1942, 2001
Johansson S, Lindholm P, Gullbo J, Larsson R, Bohlin L, Claeson P, Cytotoxicity of digitoxin and related cardiac glycosides in human tumor cells Anticancer Drugs 12:475–483, 2001
Kawamura A, Abrell LM, Maggiali F, Berova N, Nakanishi K, Labutti J, Magil S, Haupert GT, Jr., Hamlyn JM, Biological implication of conformational flexibility in ouabain: observations with two ouabain phosphate isomers Biochemistry 40:5835–5844, 2001
Schwarz SM, Bostwick HE, Medow MS, Estrogen modulates ileal basolateral membrane lipid dynamics and Na+–K+-ATPase activity Am J Physiol 254:G687–694, 1988
Davis RA, Kern F, Jr., Showalter R, Sutherland E, Sinensky M, Simon FR, Alterations of hepatic Na+, K+-atpase and bile flow by estrogen: effects on liver surface membrane lipid structure and function Proc Natl Acad Sci USA 75:4130–4134, 1978
Smith DJ, Gordon ER, Role of liver plasma membrane fluidity in the pathogenesis of estrogen-induced cholestasis J Lab Clin Med 112:679–685, 1988
Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T, Effects of steroid hormones on (Na+, K+)-ATPase activity inhibition-induced amnesia on the step-through passive avoidance task in gonadectomized mice Pharmacol Res 49:151–159, 2004
Baker ME, Computer-based search for steroid and DNA binding sites on estrogen and glucocorticoid receptors Biochem Biophys Res Commun 139:281–286, 1986
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H, Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance J Biol Chem 276:9817–9824, 2001
Ivanova T, Mendez P, Garcia-Segura LM, Beyer C, Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen J Neuroendocrinol 14:73–79, 2002
Liao JK, Cross-coupling between the oestrogen receptor and phosphoinositide 3-kinase Biochem Soc Trans 31:66–70, 2003
Zhang Z, Kumar R, Santen RJ, Song RX, The role of adapter protein Shc in estrogen non-genomic action Steroids 69:523–529, 2004
Freyberger A, Schmuck G, Screening for estrogenicity and anti-estrogenicity: a critical evaluation of an MVLN cell-based transactivation assay Toxicol Lett 155:1–13, 2005
Blaustein MP, Robinson SW, Gottlieb SS, Balke CW, Hamlyn JM, Sex, digitalis, and the sodium pump Mol Interv 3:68–72, 50, 2003
Palacios J, Marusic ET, Lopez NC, Gonzalez M, Michea L, Estradiol-induced expression of N(+)–K(+)-ATPase catalytic isoforms in rat arteries: gender differences in activity mediated by nitric oxide donors Am J Physiol Heart Circ Physiol 286:H1793–H1800, 2004
Getsios S, Chen GT, Stephenson MD, Leclerc P, Blaschuk OW, MacCalman CD, Regulated expression of cadherin-6 and cadherin-11 in the glandular epithelial and stromal cells of the human endometrium Dev Dyn 211:238–247, 1998
Chen GT, Getsios S, MacCalman CD, 17Beta-estradiol potentiates the stimulatory effects of progesterone on cadherin-11 expression in cultured human endometrial stromal cells Endocrinology 139:3512–3519, 1998
Chen GT, Getsios S, MacCalman CD, Cadherin-11 is a hormonally regulated cellular marker of decidualization in human endometrial stromal cells Mol Reprod Dev 52:158–165, 1999
Chen GT, Getsios S, MacCalman CD, Antisteroidal compounds and steroid withdrawal down-regulate cadherin-11 mRNA and protein expression levels in human endometrial stromal cells undergoing decidualisation in vitro Mol Reprod Dev 53:384–393, 1999
Gorodeski GI, Vaginal-cervical epithelial permeability decreases after menopause Fertil Steril 76:753–761, 2001
Zeng R, Li X, Gorodeski GI, Estrogen abrogates transcervical tight junctional resistance by acceleration of occludin modulation J Clin Endocrinol Metab 89:5145–5155, 2004
Ye L, Martin TA, Parr C, Harrison GM, Mansel RE, Jiang WG, Biphasic effects of 17-beta-estradiol on expression of occludin and transendothelial resistance and paracellular permeability in human vascular endothelial cells J Cell Physiol 196:362–369, 2003
Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M, Bcl-2 expression decreases cadherin-mediated cell–cell adhesion J Cell Sci 116:3687–3700, 2003
Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S, Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins Oncogene 23:1052–1063, 2004
Sengupta K, Banerjee S, Saxena N, Banerjee SK, Estradiol-induced vascular endothelial growth factor-A expression in breast tumor cells is biphasic and regulated by estrogen receptor-alpha dependent pathway Int J Oncol 22:609–614, 2003
Schmitt M, Horbach A, Kubitz R, Frilling A, Haussinger D, Disruption of hepatocellular tight junctions by vascular endothelial growth factor (VEGF): a novel mechanism for tumor invasion J Hepatol 41:274–283, 2004
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW, SSeCKS regulates angiogenesis and tight junction formation in blood–brain barrier Nat Med 9:900–906, 2003
Tushaus L, Hopert AC, Strunck E, Schubert C, Wunsche W, Vollmer G, Estrogenic and antiestrogenic regulation of MMP-2 and MMP-13 mRNA in RUCA-I endometrial tumor cells in vitro and in vivo Cancer Lett 198:99–106, 2003
Levin ER, Integration of the extra-nuclear and nuclear actions of estrogen Mol Endocrinol,2005
Razandi M, Pedram A, Greene GL, Levin ER, Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells Mol Endocrinol 13:307–319, 1999
Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ, Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation Mol Endocrinol 16:116–127, 2002
Zhang Z, Maier B, Santen RJ, Song RX, Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation Biochem Biophys Res Commun 294:926–933, 2002
Wissink S, van der Burg B, Katzenellenbogen BS, van der Saag PT, Synergistic activation of the serotonin-1A receptor by nuclear factor-kappa B and estrogen Mol Endocrinol 15:543–552, 2001
Chu S, Nishi Y, Yanase T, Nawata H, Fuller PJ, Transrepression of estrogen receptor beta signaling by nuclear factor-kappab in ovarian granulosa cells Mol Endocrinol 18:1919–1928, 2004
Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW, Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia Brain Res 1008:147–154, 2004
Scheiner-Bobis G, Schoner W, A fresh facet for ouabain action Nat Med 7:1288–1289, 2001
Wu JT, Kral JG, The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy J Surg Res 123:158–169, 2005
Ditsworth D, Zong WX: NF-kappaB.: key mediator of inflammation-associated cancer. Cancer Biol Ther 3, 2004
Mann DA, Oakley F, NF-kappaB.: a signal for cancer J Hepatol 42:610–611, 2005
Getsios S, Chen GT, Huang DT, MacCalman CD, Regulated expression of cadherin-11 in human extravillous cytotrophoblasts undergoing aggregation and fusion in response to transforming growth factor beta 1 J Reprod Fertil 114:357–363, 1998
Gorodeski GI, Estrogen biphasic regulation of paracellular permeability of cultured human vaginal-cervical epithelia J Clin Endocrinol Metab 86:4233–4243, 2001
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JQ., Contreras, R.G., Wang, R. et al. Sodium/potasium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti- breast cancer drugs?. Breast Cancer Res Treat 96, 1–15 (2006). https://doi.org/10.1007/s10549-005-9053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-005-9053-3