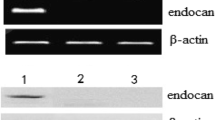
Abstract
A breast cancer-associated antigen, mammaglobin-A, is specifically expressed in 80% of primary breast tumors. The definition of immune responses against this highly expressed breast cancer-specific antigen should be of great value in the development of new therapeutic strategies for breast cancer. Thus, the purpose of this study was to identify HLA-A2-restricted mammaglobin-A-derived epitopes recognized by CD8+ cytotoxic T lymphocytes (CTL). We identified seven mammaglobin-A-derived candidate epitopes that bind the HLA-A2 molecule (Mam-A2.1-7) by means of a HLA class I-peptide binding computer algorithm from the Bioinformatics & Molecular Analysis Section of the National Institutes of Health. Subsequently, we determined that CD8+ CTLs from breast cancer patients reacted to the Mam-A2.1 (83–92, LIYDSSLCDL), Mam-A2.2 (2–10, KLLMVLMLA), Mam-A2.3 (4–12, LMVLMLAAL), Mam-A2.4 (66–74, FLNQTDETL), and Mam-A2.7 (32–40, TINPQVSKT) epitopes using an IFN-c ELISPOT assay. Interestingly, healthy individuals also showed high reactivity to the Mam-A2.2 epitope. Two CD8+ CTL lines generated in vitro against TAP-deficient T2 cells loaded with the candidate epitopes showed significant cytotoxic activity against the Mam-A2.1-4 epitopes. These CD8+CTL lines recognized a HLA-A2+breast cancer cell line expressing the Mam-A2.1 epitope. In addition, DNA vaccination of HLA-A2+/human CD8+ double-transgenic mice with a DNA construct encoding the Mam-A2.1 epitope and the HLA-A2 molecule induced a significant expansion of epitope-specific CD8+ CTLs that recognize the same HLA- A2+/Mam-A2.1+ breast cancer cell line. In conclusion, these results demonstrate the immunotherapeutic potential of mammaglobin-A for the treatment and prevention of breast cancer.
Similar content being viewed by others
References
Finke JH, Rayman P, Alexander J, Edinger M, Tubbs RR, Connelly R, Pontes E, Bukowski R: Characterization of the cytolytic activity of CD4+and CD8+tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Res 50: 2363–2370, 1990
Peoples GE, Schoof DD, Andrews JV, Goedegebuure PS, Eberlein TJ: T cell recognition of ovarian cancer. Surgery 114: 227–234, 1993
Topalian S, Solomon D, Rosenberg SA: Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol 142: 3714–3725, 1989
Linehan DC, Goedegebuure PS, Peoples GE, Rogers SO, Eberlein TJ: Tumor-specific and HLA-A2–restricted cytolysis by tumor associated lymphocytes in human metastatic breast cancer. J Immunol 155: 4486–4491, 1995
Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ: Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu derived peptide. Proc Natl Acad Sci USA 92: 432–436, 1995
Boon T, van der Bruggen P: Human tumor antigens recognized by T lymphocytes. J Exp Med 183: 725–729, 1996
Boon T, Coulie PG, Van den Eynde B: Tumor antigens recognized by T cells. Immunol Today 18: 267–268, 1997
Apostolopoulos V, Sandrin MS, McKenzie IF: Carbohydrate/peptide mimics: effect on MUC1 cancer immunotherapy. J Mol Med 77: 427–436, 1999
Finn OJ, Jerome KR, Henderson RA, Pecher G, Domenech N, Magarian-Blander J, Barratt-Boyes SM: MUC1 epithelial tumor-mucin-based immunity and cancer vaccines. Immunol Rev 145: 61–89, 1995
Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee H-G, Kanz L, Brugger W: Identification of HLA-A2–restricted T cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood 93: 4309–4317, 1999
Domenech N, Henderson RA, Finn OJ: Identification of an HLA-A11–restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol 155: 4766–4774, 1995
Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W: Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96: 3102–3108, 2002
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ulrich A, Press MF: Studies of the Her-2/neu protooncogene in human breast and ovarian cancer. Science 244: 707–712, 1989
Lohrisch C, Piccart M: HER2/neu as a predictive factor in breast cancer. Clin Breast Cancer 2: 129–135, 2001
Graham RA, Burchell JM, Taylor-Papadimitriou J: The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother 42: 71–80, 1996
Apostolopoulos V, Pietersz GA, McKenzie IF: MUC1 and breast cancer. Curr Opin Mol Ther 1: 98–103, 1999
Rahn JJ, Dabbagh L, Pasdar M, Hugh JC: The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 91: 1973–1982, 2001
Watson MA, Fleming TP: Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res 54: 4598–4602, 1994
Watson MA, Fleming TP: Mammaglobin, a mammary specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res 56: 860–865, 1996
Watson MA, Darrow C, Zimonjic DB, Popescu NC, Fleming TP: Structure and transcriptional regulation of the human mammaglobin gene, a breast cancer associated member of the uteroglobin gene family localized to chromosome 11q13. Oncogene 16: 817–824, 1998
Fleming TP, Watson MA: Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann NY Acad Sci 923: 78–89, 2000
Marchetti A, Buttitta F, Bertacca G, Zavaglia K, Bevilacqua G, Angelucci D, Viacava P, Naccarato A, Bonadio A, Barassi F, Felicioni L, Salvatore S, Mucilli F: mRNA markers of breast cancer nodal metastases: comparison between mammaglobin and carcinoembryonic antigen in 248 patients. J Pathol 195: 186–190, 2001
Grunewald K, Haun M, Urbanek M, Fiegel M, Muller-Holzner E, Gunsilius E, Dunser M, Marth C, Castle G: Mammaglobin gene expression: a superior marker of breast cancer cells in peripheral blood in comparison to epidermal growth factor receptor and cytokeratin-19. Lab Invest 80: 1071–1077, 2000
Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, Fleming TP: Mammaglobin expression in primary, metastatic and occult breast cancer. Cancer Res 59: 3028–3031, 1999
Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T: Identification of HLA-A3–restricted CD8+T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer 102: 499–506, 2002
Tanaka Y, Amos KD, Fleming TP, Eberlein TJ, Goedegebuure PS: Mammaglobin-A is a tumor-associated antigen in human breast carcinoma. Surgery 133: 74–80, 2003
Man S, Newberg MH, Crotzer VL, Luckey CJ, Williams NS, Chen Y, Huczko EL, Ridge JP, Engelhard VH: Definition of a human T cell epitope from influenza A non-structural protein 1 using HLA-A2.1 transgenic mice. Int Immunol 7: 597–605, 1995
Lustgarten J, Theobald M, Labadie C, LaFace D, Peterson P, Disis ML, Cheever MA, Sherman LA: Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD.8. Hum Immunol 52: 109–118, 1997
Parker KC, Bednarek MA, Coligan JE: Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol 152: 163–175, 1994
Bednarek MA, Sauma SY, Gammon MC, Porter G, Tamhankar S, Williamson AR, Zweerink HJ: The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J Immunol 147: 4047–4053, 1991
Yu YYL, Netuschil N, Lybarger L, Connolly JM, Hansen TH: Single-chain trimer of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol 168: 3145–3149, 2002
Lybarger L, Yu YY, Miley MJ, Fremont DH, Myers N, Primeau T, Truscott SM, Connolly JM, Hansen TH: Enhanced immune presentation of a single-chain major histocompatibility complex class I molecule engineered to optimize linkage of a C-terminally extended peptide. J Biol Chem 278: 27105–27111, 2003
Kuhns JJ, Batalia MA, Tan S, Collins EJ: Poor binding of a HER-2/neu epitope (GP2)to HLA-A2.1 is due to a lack of interactions with the center of the peptide. J Biol Chem 274: 36422–36427, 1999
Tanaka Y, Amos KD, Joo HG, Eberlein TJ, Goedegeburre PS: Modification of the HER-2/neu-derived tumor antigen GP2 improves induction of GP2–reactive cytotoxic T lymphocytes. Int J Cancer 94: 540–544, 2001
Bullock TN, Mullins DW, Colella TA, Engelhard VH: Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+)T cells for melanoma immuno-therapy in human MHC class I-transgenic mice. J Immunol 167: 5824–5831, 2001
Nijman HW, Houbiers JG, van der Burg SH, Vierboom MP, Kenemans P, Kast WM, Melief CJ: Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p 53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immun-other 14: 121–126, 1993
Manna PP, Jaramillo A, Majumder K, Campbell LG, Fleming TP, Dietz JR, DiPersio JF, Mohanakumar T: Generation of CD8+cytotoxic T lymphocytes against breast cancer cells by stimulation with mammaglobin-A-pulsed dendritic cells. Breast Cancer Res Treat 79: 133–136, 2003
Luckey CJ, Marto JA, Partridge M, Hall E, White FM, Lippolis JD, Shabanowitz J, Hunt DF, Engelhard VH: Differences in the expression of human class I MHC alleles and their associated peptides in the presence of proteosome inhibitors. J Immunol 167: 1212–1221, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jaramillo, A., Narayanan, K., Campbell, L.G. et al. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes. Breast Cancer Res Treat 88, 29–41 (2004). https://doi.org/10.1007/s10549-004-8918-1
Issue Date:
DOI: https://doi.org/10.1007/s10549-004-8918-1