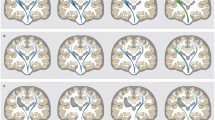
Abstract
Upper extremity (UE) impairments in infants with cerebral palsy (CP) result from reduced quality of motor experiences and “noisy” sensory inputs. We hypothesized that a neuroscience-based multi-component intervention would improve somatosensory processing and motor measures of more-affected (UEs) in infants with CP and asymmetric UE neurologic impairments, while remaining safe for less-affected UEs. Our randomized controlled trial compared infants (6–24 months) with CP receiving intervention (N = 37) versus a waitlisted group (N = 36). Treatment effects tested a direct measurement of reach smoothness (3D-kinematics), a measure of unimanual fine motor function (Bayley unimanual fine motor raw scores), and EEG measures of cortical somatosensory processing. The four-week therapist-directed, parent-administered intervention included daily (1) bimanual play; (2) less-affected UE wearing soft-constraint (6 h/day, electronically-monitored); (3) reach training on more-affected UE; (4) graduated motor-sensory training; and (5) parent education. Waitlist infants received only bimanual play. Effectiveness and safety were tested; z-scores from 54 posttest-matched typically-developing infants provided benchmarks for treatment effects. Intervention and waitlist infants had no pretest differences. Median weekly constraint wear was 38 h; parent-treatment fidelity averaged > 92%. On the more affected side, the intervention significantly increased smoothness of reach (Cohen’s d = − 0.90; p < .001) and unimanual fine motor skill (d = 0.35; p = .004). Using unadjusted p values, intervention improved somatosensory processing (d = 0.53; p = .04). All intervention effects referenced well to typically developing children. Safety of the intervention was demonstrated through positive- or non-effects on measurements involving the constrained, less-affected UE and gross motor function; unexpected treatment effects on reach smoothness occurred in less-affected UEs (d = − 0.85; p = .01). This large clinical trial demonstrated intervention effectiveness and safety for developing sensory and motor systems with improvements in reach smoothness, and developmental abilities.
Clinical Trail Registration: ClinicalTrials.gov NCT02567630, registered October 5, 2015.


Similar content being viewed by others
Data Availability
All reasonable requests from qualified scientists for unique research resources (ERP paradigms, protocols and expertise) developed with NIH funds for research purposes will be honored. We will fill requests in a timely manner. We will adhere to the NIH Grant Policy on Sharing of Unique Research Resources including the Sharing of Biomedical Research Resources Principle and Guidelines for Recipients of NIH Grants and Contracts. There is no unique biological information that could be made available to the scientific community. De-identified ERP raw data will be retained on the Abigail Wexner Research Institute at Nationwide Children’s Hospital server, and assessment data will be retained in REDCap. These data will be made available to investigators who make specific inquiry for good cause 5 years after the conclusion of the final outcomes.
References
Aarts PB, Jongerius PH, Geerdink YA, van Limbeek J, Geurts AC (2011) Modified constraint-induced movement therapy combined with bimanual training (mCIMT-BiT) in children with unilateral spastic cerebral palsy: how are improvements in arm-hand use established? Res Dev Disabil 32:271–279. https://doi.org/10.1016/j.ridd.2010.10.008
Auld ML, Boyd R, Moseley GL, Ware R, Johnston LM (2012) Tactile function in children with unilateral cerebral palsy compared to typically developing children. Disabil Rehabil 34:1488–1494. https://doi.org/10.3109/09638288.2011.650314
Bakker H, de Graaf-Peters VB, van Eykern LA, Otten B, Hadders-Algra M (2010) Development of proximal arm muscle control during reaching in young infants: from variation to selection. Infant Behav Dev 33:30–38. https://doi.org/10.1016/j.infbeh.2009.10.006
Barratt W (2006) The Barratt Simplified Measure of Social Status (BSMSS). https://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html. Accessed 28 Aug 2019
Bayley N (2006) Bayley Scales of infant and toddler development, 3rd edn. Harcourt, San Antonio
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Berry JG, Glader L, Stevenson RD, Hasan F, Crofton C, Hussain K, Hall M (2018) Associations of coexisting conditions with healthcare spending for children with cerebral palsy. J Pediatr 200:111–117.e1. https://doi.org/10.1016/j.jpeds.2018.04.021
Bhat A, Heathcock J, Galloway JC (2005) Toy-oriented changes in hand and joint kinematics during the emergence of purposeful reaching. Infant Behav Dev 28:445–465. https://doi.org/10.1016/j.infbeh.2005.03.001
Bleyenheuft Y, Dricot L, Gilis N, Kuo HC, Grandin C, Bleyenheuft C, Gordon AM, Friel KM (2015) Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: a combined DTI, TMS and fMRI pilot study. Res Dev Disabil 43–44:136–149. https://doi.org/10.1016/j.ridd.2015.06.014
Boxum AG, La Bastide-Van GS, Dijkstra LJ, Hamer EG, Hielkema T, Reinders-Messelink HA, Hadders-Algra M (2017) Development of the quality of reaching in infants with cerebral palsy: a kinematic study. Dev Med Child Neurol 59:1164–1173. https://doi.org/10.1111/dmcn.13538
Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, Carter AR, Leff AP, Copland DA, Carey LM, Cohen LG, Basso DM, Maguire JM, Cramer SC (2017a) Biomarkers of stroke recovery: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair 31:864–876. https://doi.org/10.1177/1545968317732680
Boyd RN, Ziviani J, Sakzewski L, Novak I, Badawi N, Pannek K, Elliott C, Greaves S, Guzzetta A, Whittingham K, Valentine J, Morgan C, Wallen M, Eliasson AC, Findlay L, Ware R, Fiori S, Rose S (2017b) REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open 7:e017204. https://doi.org/10.1136/bmjopen-2017-017204
Burzi V, Tealdi G, Boyd RN, Guzzetta A (2016) Action observation in infancy: implications for neuro-rehabilitation. Dev Med Child Neurol 58(Suppl 4):74–77. https://doi.org/10.1111/dmcn.13048
Byrne R, Noritz G, Maitre NL, Group NCHED (2017) Implementation of early diagnosis and intervention guidelines for cerebral palsy in a high-risk infant follow-up clinic. Pediatr Neurol 76:66–71. https://doi.org/10.1016/j.pediatrneurol.2017.08.002
Catmur C (2013) Sensorimotor learning and the ontogeny of the mirror neuron system. Neurosci Lett 540:21–27. https://doi.org/10.1016/j.neulet.2012.10.001
Chorna O, Emery L, Hamm E, Moore-Clingenpeel M, Shrivastava H, Miller A, Richard C, Maitre NL (2019) Standardized music therapy with and without acclimatization, to improve EEG data acquisition in young children with and without disability. J Neurosci Methods 321:12–19. https://doi.org/10.1016/j.jneumeth.2019.02.013
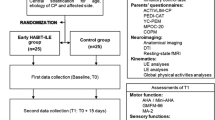
Chorna O, Heathcock J, Key A, Noritz G, Carey H, Hamm E, Nelin MA, Murray M, Needham A, Slaughter JC, Maitre NL (2015) Early childhood constraint therapy for sensory/motor impairment in cerebral palsy: a randomised clinical trial protocol. BMJ Open 5:e010212. https://doi.org/10.1136/bmjopen-2015-010212
Couper J (2002) Prevalence of childhood disability in rural KwaZulu-Natal. S Afr Med J 92:549–552
Cruz M, Jenkins R, Silberberg D (2006) The burden of brain disorders. Science 312:53. https://doi.org/10.1126/science.312.5770.53b
Damiano DL (2014) Effects of motor activity on brain and muscle development in cerebral palsy. In: Shepherd RB (ed) Cerebral palsy in infancy. Elsevier, London, pp 189–198. https://doi.org/10.1016/b978-0-7020-5099-2.00009-1
Dusing SC, Marcinowski EC, Rocha N, Tripathi T, Brown SE (2019) Assessment of parent-child interaction is important with infants in rehabilitation and can use high-tech or low-tech methods. Phys Ther 99:658–665. https://doi.org/10.1093/ptj/pzz021
Eliasson AC, Krumlinde-sundholm L, Shaw K, Wang C (2005) Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: an adapted model. Dev Med Child Neurol 47:266–275. https://doi.org/10.1017/s0012162205000502
Eliasson AC, Krumlinde-Sundholm L, Gordon AM, Feys H, Klingels K, Aarts PB, Rameckers E, Autti-Ramo I, Hoare B, European network for Health Technology A (2014) Guidelines for future research in constraint-induced movement therapy for children with unilateral cerebral palsy: an expert consensus. Dev Med Child Neurol 56:125–137. https://doi.org/10.1111/dmcn.12273
Eliasson AC, Nordstrand L, Ek L, Lennartsson F, Sjöstrand L, Tedroff K, Krumlinde-Sundholm L (2018) The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res Dev Disabil 72:191–201. https://doi.org/10.1016/j.ridd.2017.11.006
Eunson P (2012) Aetiology and epidemiology of cerebral palsy. Paediatr Child Health 22:361–366. https://doi.org/10.1016/j.paed.2012.05.008
Fallang B, Saugstad OD, Grogaard J, Hadders-Algra M (2003) Kinematic quality of reaching movements in preterm infants. Pediatr Res 53:836–842. https://doi.org/10.1203/01.PDR.0000058925.94994.BC
Ferre CL, Babik I, Michel GF (2010) Development of infant prehension handedness: a longitudinal analysis during the 6- to 14-month age period. Infant Behav Dev 33:492–502. https://doi.org/10.1016/j.infbeh.2010.06.002
Ferre CL, Carmel JB, Flamand VH, Gordon AM, Friel KM (2020) Anatomical and functional characterization in children with unilateral cerebral palsy: an atlas-based analysis. Neurorehabil Neural Repair 34:148–158. https://doi.org/10.1177/1545968319899916
Friel KM, Martin JH (2005) Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol 485:43–56. https://doi.org/10.1002/cne.20483
Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, Petra E, Chinnan A, Charles JR (2011) Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Repair 25:692–702. https://doi.org/10.1177/1545968311402508
Gulde P, Hermsdörfer J (2018) Smoothness metrics in complex movement tasks. Front Neurol 9:615. https://doi.org/10.3389/fneur.2018.00615
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
Hay K, Nelin M, Carey H, Chorna O, Moore-Clingenpeel Ma Mas M, Maitre N, Group NCHED (2018) Hammersmith infant neurological examination asymmetry score distinguishes hemiplegic cerebral palsy from typical development. Pediatr Neurol 87:70–74. https://doi.org/10.1016/j.pediatrneurol.2018.07.002
Hernández-Pérez R, Rojas-Hortelano E, de Lafuente V (2020) Integrating somatosensory information over time. Neuroscience 433:72–80. https://doi.org/10.1016/j.neuroscience.2020.02.037
Hoare B, Eliasson AC (2014) Evidence to practice commentary: upper limb constraint in infants: important perspectives on measurement and the potential for activity-dependent withdrawal of corticospinal projections. Phys Occup Ther Pediatr 34:22–25. https://doi.org/10.3109/01942638.2014.868662
Hollung SJ, Bakken IJ, Vik T, Lydersen S, Wiik R, Aaberg KM, Andersen GL (2020) Comorbidities in cerebral palsy: a patient registry study. Dev Med Child Neurol 62:97–103. https://doi.org/10.1111/dmcn.14307
Hoon AH Jr, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV (2009) Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51:697–704. https://doi.org/10.1111/j.1469-8749.2009.03306.x
Ismail FY, Fatemi A, Johnston MV (2017) Cerebral plasticity: windows of opportunity in the developing brain. Eur J Paediatr Neurol 21:23–48. https://doi.org/10.1016/j.ejpn.2016.07.007
Jackman M, Lannin N, Galea C, Sakzewski L, Miller L, Novak I (2020) What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. Aust Occup Ther J 67:269–280. https://doi.org/10.1111/1440-1630.12666
Koenig T, Melie-García L (2010) A method to determine the presence of averaged event-related fields using randomization tests. Brain Topogr 23:233–242. https://doi.org/10.1007/s10548-010-0142-1
Körding KP, Wolpert DM (2006) Bayesian decision theory in sensorimotor control. Trends Cogn Sci 10:319–326. https://doi.org/10.1016/j.tics.2006.05.003
Kuban KC, Allred EN, O'Shea M, Paneth N, Pagano M, Leviton A, Group ESCP-A (2008) An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr 153:466–472. https://doi.org/10.1016/j.jpeds.2008.04.013
Kuo HC, Gordon AM, Henrionnet A, Hautfenne S, Friel KM, Bleyenheuft Y (2016) The effects of intensive bimanual training with and without tactile training on tactile function in children with unilateral spastic cerebral palsy: a pilot study. Res Dev Disabil 49–50:129–139. https://doi.org/10.1016/j.ridd.2015.11.024
Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW (2014) Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol 56:1072–1077. https://doi.org/10.1111/dmcn.12513
Lowes LP, Mayhan M, Orr T, Batterson N, Tonneman JA, Meyer A, Alfano L, Wang W, Whalen CN, Nelin MA, Lo WD, Case-Smith J (2014) Pilot study of the efficacy of constraint-induced movement therapy for infants and toddlers with cerebral palsy. Phys Occup Ther Pediatr 34:4–21. https://doi.org/10.3109/01942638.2013.810186
Machado LR, Heathcock J, Carvalho RP, Pereira ND, Tudella E (2019) Kinematic characteristics of arm and trunk when drinking from a glass in children with and without cerebral palsy. Clin Biomech 63:201–206. https://doi.org/10.1016/j.clinbiomech.2019.03.011
Maitre NL, Barnett ZP, Key AP (2012) Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol 27:1276–1283. https://doi.org/10.1177/0883073811435682
Maitre NL, Key AP, Chorna OD, Slaughter JC, Matusz PJ, Wallace MT, Murray MM (2017) The dual nature of early-life experience on somatosensory processing in the human infant brain. Curr Biol 27:1048–1054. https://doi.org/10.1016/j.cub.2017.02.036
Maitre NL, Burton VJ, Duncan AF, Iyer S, Ostrander B, Winter S, Ayala L, Burkhardt S, Gerner G, Getachew R, Jiang K, Lesher L, Perez CM, Moore-Clingenpeel M, Lam R, Lewandowski DJ, Byrne R (2020) Network implementation of guideline for early detection decreases age at cerebral palsy diagnosis. Pediatrics 145:e20192126. https://doi.org/10.1542/peds.2019-2126
Matusz PJ, Key AP, Gogliotti S, Pearson J, Auld ML, Murray MM, Maitre NL (2018) Somatosensory plasticity in pediatric cerebral palsy following constraint-induced movement therapy. Neural Plast 2018:1891978. https://doi.org/10.1155/2018/1891978
Needham AW, Wiesen SE, Hejazi JN, Libertus K, Christopher C (2017) Characteristics of brief sticky mittens training that lead to increases in object exploration. J Exp Child Psychol 164:209–224. https://doi.org/10.1016/j.jecp.2017.04.009
Nevalainen P, Lauronen L, Pihko E (2014) Development of human somatosensory cortical functions—what have we learned from magnetoencephalography: a review. Front Hum Neurosci 8:158. https://doi.org/10.3389/fnhum.2014.00158
NICHD (2019) Neonatal Research Network (NRN). Eunice Kennedy Shriver NICHD Neonatal Research Network https://www.nichd.nih.gov/research/supported/nrn.. Accessed 20 Oct 2019
NIH-RePORT (2018) Multisite RCT of 3 neurorehabilitation therapies for infants with asymmetrical CP. NIH research report online reporting tools (RePORT). https://projectreporter.nih.gov/project_info_description.cfm?aid=9228376&icde=46839437. Accessed 25 Sept 2019
NIH-RePORT (2019) Perinatal Arterial stroke: a multi-site RCT of intensive infant rehabilitation (I-ACQUIRE). NIH research report online reporting tools (RePORT). https://projectreporter.nih.gov/project_info_description.cfm?aid=9662340&icde=46839262. Accessed 25 Sept 2019
NINDS (2013) Cerebral palsy: hope through research. National institute of neurological disorders and stroke. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Cerebral-Palsy-Hope-Through-Research#3104_24. Accessed 20 Apr 2020
Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, Eliasson AC, de Vries LS, Einspieler C, Fahey M, Fehlings D, Ferriero DM, Fetters L, Fiori S, Forssberg H, Gordon AM, Greaves S, Guzzetta A, Hadders-Algra M, Harbourne R, Kakooza-Mwesige A, Karlsson P, Krumlinde-Sundholm L, Latal B, Loughran-Fowlds A, Maitre N, McIntyre S, Noritz G, Pennington L, Romeo DM, Shepherd R, Spittle AJ, Thornton M, Valentine J, Walker K, White R, Badawi N (2017) Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 171:897–907. https://doi.org/10.1001/jamapediatrics.2017.1689
Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, Langdon K, Namara MM, Paton MCB, Popat H, Shore B, Khamis A, Stanton E, Finemore OP, Tricks A, te Velde A, Dark L, Morton N, Badawi N (2020) State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep 20:3. https://doi.org/10.1007/s11910-020-1022-z
Schneiberg S, McKinley P, Gisel E, Sveistrup H, Levin MF (2010) Reliability of kinematic measures of functional reaching in children with cerebral palsy. Dev Med Child Neurol 52:e167–173. https://doi.org/10.1111/j.1469-8749.2010.03635.x
Schneider DM (2020) Reflections of action in sensory cortex. Curr Opin Neurobiol 64:53–59. https://doi.org/10.1016/j.conb.2020.02.004
Sofroniew NJ, Vlasov YA, Hires SA, Freeman J, Svoboda K (2015) Neural coding in barrel cortex during whisker-guided locomotion. eLife 4:e12559. https://doi.org/10.7554/eLife.12559
Taub E, Ramey SL, DeLuca S, Echols K (2004) Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics 113:305–312. https://doi.org/10.1542/peds.113.2.305
Tzovara A, Murray MM, Michel CM, De Lucia M (2012) A tutorial review of electrical neuroimaging from group-average to single-trial event-related potentials. Dev Neuropsychol 37:518–544. https://doi.org/10.1080/87565641.2011.636851
Volpe JJ (2009) The encephalopathy of prematurity—brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol 16:167–178. https://doi.org/10.1016/j.spen.2009.09.005
Whittingham K, Sanders MR, McKinlay L, Boyd RN (2016) Parenting intervention combined with acceptance and commitment therapy: a trial with families of children with cerebral palsy. J Pediatr Psychol 41:531–542. https://doi.org/10.1093/jpepsy/jsv118
Acknowledgements
We would like to thank all our patients and their families as well as Ms. Joanna Kinner for her administrative assistance throughout this project. NLM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was supported by National Institutes of Child Health and Human Development, grant number R01HD081120-01A1 to NLM. MMM is supported by the Swiss National Science Foundation (169206). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BBOP group*: Stephanie Burkhardt, MPH; Lelia Emery, MMT; Kaleigh Hague, MA; Katelyn Levengood, DPT; Dennis J. Lewandowski, PhD; Mary Ann Nelin, MD, FAAP; Caitlin Pennington; Lindsay Pietruszewski, DPT; Jessica Purnell; Briana Sowers. * Center for Perinatal Research at the Abigail Wexner Research Institute, Nationwide Children’s Hospital, Columbus, OH, USA.
Funding
This study was supported by National Institutes of Child Health and Human Development, Grant Number 1 R01 HD081120-01A1 to NLM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Contributions
NLM conceived and designed the study, acquired and analyzed the data, drafted the manuscript created the figures, and obtained funding. AJ acquired and analyzed EEG data and created figures. PJY designed the trial data analysis, analyzed data, created tables, and drafted a significant portion of the manuscript. APK helped conceptualize the study and the EEG analysis, monitored EEG data reliability, and drafted a significant portion of the manuscript. JCS contributed to the study design, analyzed data, and drafted a significant portion of the manuscript. HC acquired data, designed fidelity measures, and drafted a significant portion of the manuscript. AN helped conceptualize the study intervention, designed the sticky mittens, and drafted a significant portion of the manuscript. MMM helped conceptualize the study and the EEG analysis and drafted a significant portion of the manuscript. JH helped conceptualize the study kinematics, analyzed the kinematics data and drafted a significant portion of the manuscript and figures.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Maitre reports USPTO 29/577,142 (C-MITT, Soft Constraint Harness for Infants 6–27 months—for filing design application), pending. The remaining authors report no conflict of interest.
Ethical Approval
This research was approved by the Nationwide Children’s Hospital Institutional Review Board on June 18, 2015.
Informed Consent
Written informed consent was obtained for each subject included in the study. Consent for publication of non-identifiable results is included in the written informed consent.
Additional information
Handling Editor: Christoph M. Michel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of BBOP group are listed in Acknowledgements section.
Rights and permissions
About this article
Cite this article
Maitre, N.L., Jeanvoine, A., Yoder, P.J. et al. Kinematic and Somatosensory Gains in Infants with Cerebral Palsy After a Multi-Component Upper-Extremity Intervention: A Randomized Controlled Trial. Brain Topogr 33, 751–766 (2020). https://doi.org/10.1007/s10548-020-00790-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-020-00790-5