Abstract
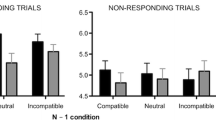
Reporting the ink color of a written word when it is itself a color name incongruent with the ink color (e.g. “red” printed in blue) induces a robust interference known as the Stroop effect. Although this effect has been the subject of numerous functional neuroimaging studies, its neuronal substrate is still a matter of debate. Here, we investigated the spatiotemporal dynamics of interference-related neural events using magnetoencephalography (MEG) and voxel-based analyses (SPM8). Evoked magnetic fields (EMFs) were acquired in 12 right-handed healthy subjects performing a color-word Stroop task. Behavioral results disclosed a classic interference effect with longer mean reaction times for incongruent than congruent stimuli. At the group level, EMFs’ differences between incongruent and congruent trials spanned from 380 to 700 ms post-stimulus onset. Underlying neural sources were identified in the left pre-supplementary motor area (pre-SMA) and in the left posterior parietal cortex (PPC) confirming the role of these regions in conflict processing.


Similar content being viewed by others
References
Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL (2002) A developmental fMRI study of the Stroop color-word task. Neuroimage 16(1):61–75
Apitz T, Bunzeck N (2012) Reward modulates the neural dynamics of early visual category processing. Neuroimage 63(3):1614–1622
Appelbaum LG, Meyerhoff KL, Woldorff MG (2009) Priming and backward influences in the human brain: processing interactions during the stroop interference effect. Cereb Cortex 19(11):2508–2521
Badzakova-Trajkov G, Barnett KJ, Waldie KE, Kirk IJ (2009) An ERP investigation of the Stroop task: the role of the cingulate in attentional allocation and conflict resolution. Brain Res 1253:139–148
Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R (2000a) fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci 12(6):988–1000
Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Barad V, Gullett D, Shah C, Brown C (2000b) Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cogn Brain Res 10(1–2):1–9
Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001) Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11(9):837–848
Bayless SJ, Gaetz WC, Cheyne DO, Taylor MJ (2006) Spatiotemporal analysis of feedback processing during a card sorting task using spatially filtered MEG. Neurosci Lett 410(1):31–36
Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ (1993) Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 31(9):907–922
Bialystok E, Craik FI, Grady C, Chau W, Ishii R, Gunji A, Pantev C (2005) Effect of bilingualism on cognitive control in the Simon task: evidence from MEG. Neuroimage 24(1):40–49
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108(3):624–652
Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD (2002) Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17(3):1562–1571
Bunzeck N, Doeller CF, Fuentemilla L, Dolan RJ, Duzel E (2009) Reward motivation accelerates the onset of neural novelty signals in humans to 85 milliseconds. Curr Biol 19(15):1294–1300
Bunzeck N, Guitart-Masip M, Dolan RJ, Duzel E (2011) Contextual novelty modulates the neural dynamics of reward anticipation. J Neurosci 31(36):12816–12822
Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 6(4):270–282
Carrette E, Op de Beeck M, Bourguignon M, Boon P, Vonck K, Legros B, Goldman S, Van Bogaert P, De Tiege X (2011) Recording temporal lobe epileptic activity with MEG in a light-weight magnetic shield. Seizure 20(5):414–418
Carter CS, Mintun M, Cohen JD (1995) Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage 2(4):264–272
Carter CS, Mintun M, Nichols T, Cohen JD (1997) Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 154(12):1670–1675
Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280(5364):747–749
Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000) Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97(4):1944–1948
Chen S, Melara RD (2009) Sequential effects in the Simon task: conflict adaptation or feature integration? Brain Res 1297:89–100
Chen A, Bailey K, Tiernan BN, West R (2011) Neural correlates of stimulus and response interference in a 2–1 mapping stroop task. Int J Psychophysiol 80(2):129–138
Coderre E, Conklin K, van Heuven WJ (2011) Electrophysiological measures of conflict detection and resolution in the Stroop task. Brain Res 1413:51–59
Cohen JD, Dunbar K, McClelland JL (1990) On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev 97(3):332–361
Cohen JD, Botvinick M, Carter CS (2000) Anterior cingulate and prefrontal cortex: who’s in control? Nat Neurosci 3(5):421–423
Coulthard EJ, Nachev P, Husain M (2008) Control over conflict during movement preparation: role of posterior parietal cortex. Neuron 58(1):144–157
De Tiège X, Op de Beeck M, Funke M, Legros B, Parkkonen L, Goldman S, Van Bogaert P (2008) Recording epileptic activity with MEG in a light-weight magnetic shield. Epilepsy Res 82(2–3):227–231
Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003) Cognitive and brain consequences of conflict. Neuroimage 18(1):42–57
Furl N, van Rijsbergen NJ, Kiebel SJ, Friston KJ, Treves A, Dolan RJ (2010) Modulation of perception and brain activity by predictable trajectories of facial expressions. Cereb Cortex 20(3):694–703
Furl N, Kumar S, Alter K, Durrant S, Shawe-Taylor J, Griffiths TD (2011) Neural prediction of higher-order auditory sequence statistics. Neuroimage 54(3):2267–2277
Garavan H, Ross TJ, Kaufman J, Stein EA (2003) A midline dissociation between error-processing and response–conflict monitoring. Neuroimage 20(2):1132–1139
George MSKTA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Hocwitz B, Herscovitch P, Post RM (1993) Regional brain activity when selecting a response despite interference: an H215O PET study of the stroop and an emotional stroop. Hum Brain Mapp 1(3):194–209
Giani AS, Ortiz E, Belardinelli P, Kleiner M, Preissl H, Noppeney U (2012) Steady-state responses in MEG demonstrate information integration within but not across the auditory and visual senses. Neuroimage 60(2):1478–1489
Goldenholz DM, Ahlfors SP, Hamalainen MS, Sharon D, Ishitobi M, Vaina LM, Stufflebeam SM (2009) Mapping the signal-to-noise-ratios of cortical sources in magnetoencephalography and electroencephalography. Hum Brain Mapp 30(4):1077–1086
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15(1):20–25
Hämäläinen MS (1995) Functional localization based on measurements with a whole-head magnetometer system. Brain Topogr 7(4):283–289
Hämäläinen MS, Ilmoniemi R (1994) Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput 32(1):35–42
Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV (1993) Magnetoencephalography, theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65(2):413–497
Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W (2008) The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci 20(2):215–225
Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004) Anterior cingulate conflict monitoring and adjustments in control. Science 303(5660):1023–1026
Kiehl KA, Liddle PF, Hopfinger JB (2000) Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 37(2):216–223
Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K (2012) Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One 7(8):e43957
Larson MJ, Kaufman DA, Perlstein WM (2009) Conflict adaptation and cognitive control adjustments following traumatic brain injury. J Int Neuropsychol Soc 15(6):927–937
Liotti M, Woldorff MG, Perez R, Mayberg HS (2000) An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia 38(5):701–711
Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, Kilner J, Barnes G, Oostenveld R, Daunizeau J, Flandin G, Penny W, Friston K (2011) EEG and MEG data analysis in SPM8. Comput Intell Neurosci 2011:852961
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288(5472):1835–1838
MacLeod CM (1991) Half a century of research on the Stroop effect: an integrative review. Psychol Bull 109(2):163–203
MacLeod CM, MacDonald PA (2000) Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci 4(10):383–391
Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M (2004) Prefrontal-cingulate activation during executive control: which comes first? Brain Res Cogn Brain Res 18(3):278–287
Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF (2001) The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res 12(3):467–473
Milham MP, Banich MT, Claus ED, Cohen NJ (2003) Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18(2):483–493
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66(8):811–822
Nee DE, Wager TD, Jonides J (2007) Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7(1):1–17
Neumann J, Lohmann G, Derrfuss J, von Cramon DY (2005) Meta-analysis of functional imaging data using replicator dynamics. Hum Brain Mapp 25(1):165–173
Neumann J, von Cramon DY, Lohmann G (2008) Model-based clustering of meta-analytic functional imaging data. Hum Brain Mapp 29(2):177–192
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Parkkonen L (2010) Instrumentation and data preprocessing. In: Hansen PC, Kringelbach ML, Salmelin R (eds) MEG: an introduction to methods. Oxford University Press, Oxford, pp 24–63
Perianez JA, Maestu F, Barcelo F, Fernandez A, Amo C, Ortiz-Alonso T (2004) Spatiotemporal brain dynamics during preparatory set shifting: MEG evidence. Neuroimage 21(2):687–695
Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999) An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45(10):1237–1258
Rushworth MF, Kennerley SW, Walton ME (2005) Cognitive neuroscience: resolving conflict in and over the medial frontal cortex. Curr Biol 15(2):R54–R56
Sharon D, Hämäläinen MS, Tootell RB, Halgren E, Belliveau JW (2007) The advantage of combining MEG and EEG: comparison to fMRI in focally stimulated visual cortex. Neuroimage 36(4):1225–1235
Snyder LH, Batista AP, Andersen RA (2000) Intention-related activity in the posterior parietal cortex: a review. Vision Res 40(10–12):1433–1441
Stoet G, Snyder LH (2007) Correlates of stimulus-response congruence in the posterior parietal cortex. J Cogn Neurosci 19(2):194–203
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Taulu S, Simola J, Kajola M (2005) Applications of the signal space separation method. IEEE Trans Signal Process 53(9):3359–3372
Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA (1997) Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage 6(2):81–92
Ukai S, Shinosaki K, Ishii R, Ogawa A, Mizuno-Matsumoto Y, Inouye T, Hirabuki N, Yoshimine T, Robinson SE, Takeda M (2002) Parallel distributed processing neuroimaging in the Stroop task using spatially filtered magnetoencephalography analysis. Neurosci Lett 334(1):9–12
Ullsperger M, von Cramon DY (2004) Neuroimaging of performance monitoring: error detection and beyond. Cortex 40(4–5):593–604
Van Veen V, Carter CS (2002) The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14(4):593–602
Van Veen V, Carter CS (2005) Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage 27(3):497–504
Van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001) Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14(6):1302–1308
Weil R, Kilner J, Haynes JD, Rees G (2007) Neural correlates of perceptual filling-in of an artificial scotoma in humans. Proc Natl Acad Sci USA 104(12):5211–5216
West R, Alain C (1999) Event-related neural activity associated with the Stroop task. Brain Res Cogn Brain Res 8(2):157–164
West R, Alain C (2000) Effects of task context and fluctuations of attention on neural activity supporting performance of the Stroop task. Brain Res 873(1):102–111
West R, Herndon RW, Covell E (2003) Neural correlates of age-related declines in the formation and realization of delayed intentions. Psychol Aging 18(3):461–473
West R, Jakubek K, Wymbs N, Perry M, Moore K (2005) Neural correlates of conflict processing. Exp Brain Res 167(1):38–48
West R, Choi P, Travers S (2010) The influence of negative affect on the neural correlates of cognitive control. Int J Psychophysiol 76(2):107–117
Acknowledgments
Sophie Galer (Research Fellow), Noémie Ligot (Clinical Master Specialist Applicant to a PhD) and Xavier De Tiège (Post-doctorate Clinical Master Specialist) are supported by a research grant from the Fonds de la Recherche Scientifique (FRS-FNRS, Belgium). Mathieu Bourguignon is supported by a research grant from the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA, FRS-FNRS, Belgium). Charline Urbain was supported by a special grant of the Université Libre de Bruxelles (Vigneron Foundation) and the ULB-ARC project “Pathophysiology of Memory Consolidation Processes”. This work has been supported by a research grant from the Fonds de la Recherche Scientifique (Research convention: 3.4508.10, FRS-FNRS, Belgium).
Conflict of interest
None of the authors has a potential conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galer, S., Op De Beeck, M., Urbain, C. et al. Investigating the Neural Correlates of the Stroop Effect with Magnetoencephalography. Brain Topogr 28, 95–103 (2015). https://doi.org/10.1007/s10548-014-0367-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-014-0367-5