Abstract
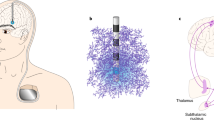
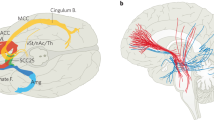
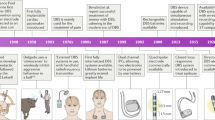
Although the appellation of personalized medicine is generally attributed to advanced therapeutics in molecular medicine, deep brain stimulation (DBS) can also be so categorized. Like its medical counterpart, DBS is a highly personalized intervention that needs to be tailored to a patient’s individual anatomy. And because of this, DBS like more conventional personalized medicine, can be highly specific where the object of care is an N = 1. But that is where the similarities end. Besides their differing medical and surgical provenances, these two varieties of personalized medicine have had strikingly different impacts. The molecular variant, though of a more recent vintage has thrived and is experiencing explosive growth, while DBS still struggles to find a sustainable therapeutic niche. Despite its promise, and success as a vetted treatment for drug resistant Parkinson’s Disease, DBS has lagged in broadening its development, often encountering regulatory hurdles and financial barriers necessary to mount an adequate number of quality trials. In this paper we will consider why DBS—or better yet neuromodulation—has encountered these challenges and contrast this experience with the more successful advance of personalized medicine. We will suggest that personalized medicine and DBS’s differential performance can be explained as a matter of timing and complexity. We believe that DBS has struggled because it has been a journey of scientific exploration conducted without a map. In contrast to molecular personalized medicine which followed the mapping of the human genome and the Human Genome Project, DBS preceded plans for the mapping of the human brain. We believe that this sequence has given personalized medicine a distinct advantage and that the fullest potential of DBS will be realized both as a cartographical or electrophysiological probe and as a modality of personalized medicine.
Similar content being viewed by others
References
AAMC Task Force on Financial Conflicts of Interest in Clinical Research (2003) Protecting subjects, preserving trust, promoting progress I: policy and guidelines for the oversight of individual financial interests in human subjects research. J Assoc Am Med Coll 78(2):225–236
Abelson, R. (2007, October 27). Medtronic, Again questioned over payments to doctors, Is subject of Senator’s Inquiry. The New York Times, New york
Andoh J, Zatorre RJ (2013) Mapping interhemispheric connectivity using functional MRI after transcranial magnetic stimulation on the human auditory cortex. Neuroimage 79C:162–171. doi:10.1016/j.neuroimage.2013.04.078
Armstrong, D. (2008, September 25). Lawsuit says Medtronic gave doctors array of perks. Wall Street J
Bernad DM (2009) Humanitarian Use Device and Humanitarian Device Exemption regulatory programs: pros and cons. Expert Rev Med Devices 6(2):137–145. doi:10.1586/17434440.6.2.137
Cashin-Garbutt A (2012) Personalized medicine and the human genome project. News-Medical. http://www.news-medical.net/news/20120601/Personalized-medicine-and-the-Human-Genome-Project.aspx. Retrieved March 15, 2013
Downing GJ (2009) Policy perspectives on the emerging pathways of personalized medicine. Dialogues Clin Neurosci 11(4):377–387
Fins JJ (2000) A proposed ethical framework for interventional cognitive neuroscience: a consideration of deep brain stimulation in impaired consciousness. Neurol Res 22(3):273–278
Fins JJ (2008) Surgical innovation and ethical dilemmas: precautions and proximity. Cleveland Clin J Med, 75(Suppl_6), S7–S12. doi: 10.3949/ccjm.75.Suppl_6.S7
Fins JJ (2009) Deep brain stimulation, free markets and the scientific commons: is it time to revisit the Bayh-Dole Act of 1980? Neuromodulation 13:153–159
Fins JJ (2012) Deep brain stimulation as a probative biology: scientific inquiry and the mosaic device. AJOB Neurosci 3(1):4–8
Fins JJ (2013) Devices, drugs & difference: deep brain stimulation and the advent of personalized medicine. In: Springer handbook on neuorethics (in press)
Fins JJ, Schachter M (2001) Investigators, industry, and the heuristic device: ethics, patent law, and clinical innovation. Account Res 8(3):219–233
Fins JJ, Schiff ND (2010) Conflicts of interest in deep brain stimulation research and the ethics of transparency. J Clin Ethics 21(2):125–132
Fins JJ, Mayberg HS, Nuttin B, Kubu CS, Galert T, Sturm V, Schlaepfer TE (2011a) Misuse of the FDA’s humanitarian device exemption in deep brain stimulation for obsessive-compulsive disorder. Health Affairs 30(2):302–311
Fins JJ, Schlaepfer TE, Nuttin B, Kubu CS, Galert T, Sturm V, Mayberg HS (2011b) Ethical guidance for the management of conflicts of interest for researchers, engineers and clinicians engaged in the development of therapeutic deep brain stimulation. J Neural Eng 8:1–6
Fins JJ, Dorfman GS, Pancrazio JJ (2012) Challenges to deep brain stimulation: a pragmatic response to ethical, fiscal, and regulatory concerns. Ann N Y Acad Sci 1265:80–90
Food and Drug Administration (2010, July 8) Guidance for HDE Holders, Institutional Review Boards (IRBs), Clinical Investigators, and FDA Staff—Humanitarian Device Exemption (HDE) Regulation: Questions and Answers. Guidance for HDE Holders, Institutional Review Boards (IRBs), Clinical Investigators, and FDA Staff. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm110194.htm. Retrieved March 15, 2013
Gitlin JM (2011) Calculating the economic impact of the Human Genome Project (Rep.). Battelle Technology Partnership Practice
Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS (2009) A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65(4):276–282
Han X (2012) In vivo application of optogenetics for neural circuit analysis. ACS Chem Neurosci 3(8):577–584
Hira R, Ohkubo F, Tanaka YR, Masamizu Y, Augustine GJ, Kasai H, Matsuzaki M (2013) In vivo optogenetic tracing of functional corticocortical connections between motor forelimb areas. Front Neural Circuits 7:55
Human Genome Management Information System (2011, July 18) About the Human Genome Project. About the Human Genome Project. http://www.ornl.gov/sci/techresources/Human_Genome/project/about.shtml. Retrieved March 15, 2013
Insel, T (2011) NIMH Director’s Blog. NIMH RSS. http://www.nimh.nih.gov/about/director/index.shtml. Retrieved March 15, 2013
Institute of Medicine. Committee on the Public Health Effectiveness of the FDA 510(k) Clearence Process (2011) Medical devices and the public’s health: The FDA 510(k) clearance process at 35 years. National Academies Press, Washington, DC
Kaplan A (2012) Without an adequate ethical infrastructure, the road to personalized medicine will be rocky at best. Clin Pharmacol Ther 92(4):411–412
Keeling P, Roth M, Zietlow T (2012, September 15) The economics of personalized medicine: commercialization as a driver of return on investment. New Biotechnol 26(6): 720–731. doi: http://dx.doi.org/10.1016/j.nbt.2012.06.001
Maglo KN (2012) Group-based and personalized care in an age of genomic and evidence-based medicine: a reappraisal. Perspect Biol Med 55(1):137–154
Markoff J (2013) Obama seeking to boost study of human brain. The New York Times. http://www.nytimes.com/2013/02/18/science/project-seeks-to-build-map-of-human-brain.html?pagewanted=all
McKinney R, Korn D (2005) Should an institution that has commercial rights in a new drug or device be allowed to evaluate the technology? PLoS Med 2(1):E9. doi:10.1371/journal.pmed.0020009
Medtronic, Inc v. Lohr (518 US 470 1996)
Ondo WG, Bronte-Stewart H (2005) The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg 83(4):142–147. doi:10.1159/000088654
Pena C, Bowsher K, Costello A, De Luca R, Doll S, Li K, Stevens T (2007) An overview of FDA medical device regulation as it relates to deep brain stimulation devices. IEEE Trans Neural Syst Rehabil Eng 15(3):421–424. doi:10.1109/TNSRE.2007.903973
Personalized Medicine Coalition. (2006). The case for personalized medicine [scholarly project]. In Personalized Medicine Coalition. http://www.personalizedmedicinecoalition.org/sites/default/files/files/PM_by_the_Numbers.pdf
Personalized Medicine Coalition. (2011). The case for personalized medicine [scholarly project]. In Personalized Medicine Coalition. http://www.personalizedmedicinecoalition.org/sites/default/files/files/PM_by_the_Numbers.pdf
Personalized Medicine Coalition. (2013). PMC membership. personalized medicine coalition. http://www.personalizedmedicinecoalition.org/members. Retrieved March 15, 2013
Schermer M (2011) Ethical issues in deep brain stimulation. Frontiers Integr Neruosci 5:17
Schiff ND, Giacino JT, Fins JJ (2009) Deep brain stimulation, neuroethics, and the minimally conscious state: moving beyond proof of principle. Arch Neurol 66(6):697–702. doi:10.1001/archneurol.2009.79
Schlaepfer TE, Fins JJ (2010) Deep brain stimulation and the neuroethics of responsible publishing: when one is not enough. J Am Med Assoc 303(8):775–776
Szalavitz M (2013) Brain map: president obama proposes first detailed guide of human brain function. Time. http://healthland.time.com/2013/02/19/brain-map-president-obama-proposes-first-detailed-guide-of-human-brain-function/. Retrieved March 14, 2013
Tufts Center for the Study of Drug Development. (2011) Lack of clinically useful diagnostics hinder growth in personalized medicines. Impact Report 13:4
U.S. Department of Health & Human Services (2006) Personalized health care. personalized health care. http://www.hhs.gov/myhealthcare/. Retrieved March 15, 2013
Willard HF, Ginsburg GS (2009) Organization, variation and expression of the human genome as a foundation of genomic and personalized medicine. In: Genomic and personalized medicine. Elsevier/Academic Press, Amsterdam, pp 4–21
Wren K (2004) How the human genome is transforming medicine. Msnbc.com. http://www.nbcnews.com/id/6291903/ns/technology_and_science-science/t/how-human-genome-transforming-medicine/. Retrieved March 15, 2013
Author information
Authors and Affiliations
Corresponding author
Additional information
This is one of several papers published together in Brain Topography on the ‘‘Special Topic: Clinical and Ethical Implications of Neuromodulation Techniques”.
Rights and permissions
About this article
Cite this article
Fins, J.J., Shapiro, Z.E. Deep Brain Stimulation, Brain Maps and Personalized Medicine: Lessons from the Human Genome Project. Brain Topogr 27, 55–62 (2014). https://doi.org/10.1007/s10548-013-0297-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-013-0297-7