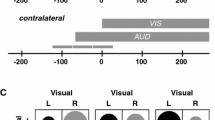
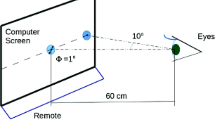
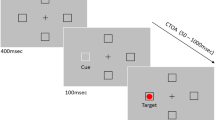
Abstract
Saccadic reaction time (SRT) to a visual target tends to be shorter when auditory stimuli are presented in close temporal and spatial proximity, even when subjects are instructed to ignore the auditory non-target (focused attention paradigm). Previous studies using pairs of visual and auditory stimuli differing in both azimuth and vertical position suggest that the amount of SRT facilitation decreases not with the physical but with the perceivable distance between visual target and auditory non-target. Steenken et al. (Brain Res 1220:150–156, 2008) presented an additional white-noise masker background of three seconds duration. Increasing the masker level had a diametrical effect on SRTs in spatially coincident versus disparate stimulus configurations: saccadic responses to coincident visual–auditory stimuli are slowed down, whereas saccadic responses to disparate stimuli are speeded up. Here we show that the time-window-of-integration model accounts for this observation by variation of a perceivable-distance parameter in the second stage of the model whose value does not depend on stimulus onset asynchrony between target and non-target.

Similar content being viewed by others
Notes
For a statistical treatment of this issue (confidence intervals), a non-parametric bootstrap method seems indicated and is planned in future work.
References
Bell AH, Corneil BD, Meredith MA, Munoz DP (2001) The influence of stimulus properties on multisensory processing in the awake primate superior colliculus. Can J Exp Psychol 55:123–132
Bell AH, Meredith A, Van Opstal AJ, Munoz DP (2005) Crossmodal integration in the primate superior colliculus underlying the preparation and initiation of saccadic eye movements. J Neurophysiol 93:3659–3673
Bell AH, Meredith MA, Van Opstal AJ, Munoz DP (2006) Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp Brain Res 174(1):53–9
Blauert J (1997) The psychophysics of human sound localization 2nd edn. MIT Press, Cambridge
Colonius H, Arndt P (2001) A two-stage model for visual–auditory interaction in saccadic latencies. Percept Psychophys 63:126–147
Colonius H, Diederich A (2004) Multisensory interaction in saccadic reaction time: a time-window-of-integration model. J Cogn Neurosci 16:1000–1009
Corneil BD, Munoz DP (1996) The influence of auditory and visual distractors on human orienting gaze shifts. J Neurosci 16:8193–8207
Diederich A, Colonius H (2004) Modeling the time course of multisensory interaction in manual and saccadic responses. In: Calvert G, Spence C, Stein BE (eds) Handbook of multisensory processes. MIT Press, Cambridge
Diederich A, Colonius H (2007a) Why two “distractors” are better than one: modeling the effect of nontarget auditory and tactile stimuli on visual saccadic reaction time. Exp Brain Res 179:43–54
Diederich A, Colonius H (2007b) Modeling spatial effects in visual-tactile saccadic reaction time. Percept Psychophys 69(1):56–67
Diederich A, Colonius H (2008a) Crossmodal interaction in saccadic reaction time: separating multisensory from warning effects in the time window of integration model. Exp Brain Res 186:1-22
Diederich A, Colonius H (2008b). When a high-intensity “distractor” is better then a low-intensity one: modeling the effect of an auditory or tactile nontarget stimulus on visual saccadic reaction time. Brain Res 1242:219–230
Diederich A, Colonius H, Schomburg A (2008) Assessing age-related multisensory enhancement with the time-window-of-integration model. Neuropsychologia 46:2556–2562
Frens MA, van Opstal (1998) Visual–auditory interactions modulate saccade-related activity in monkey superior colliculus. Brain Res Bull 46:211–224
Frens MA, Van Opstal AJ, Van der Willigen RF (1995) Spatial and temporal factors determine auditory–visual interactions in human saccadic eye movements. Percept Psychophys 57:802–816
Good MD, Gilkey RH (1996) Sound localization in noise: the effect of signal-to-noise ratio. J Acoust Soc Am 99:1108–1117
Harrington LK, Peck CK (1998) Spatial disparity affects visual–auditory interactions in human sensorimotor processing. Exp Brain Res 122:247–252
Heuermann H, Colonius H (2001) Spatial and temporal factors in visual–auditory interaction. In: Sommerfeld E, Kompass R, Lachmann T (eds) Proceedings of the 17th meeting of the international society for psychophysics. Pabst Science, Lengerich, pp 118–123
Hughes HC, Reuter-Lorenz PA, Nozawa G, Fendrich R (1994) Visual–auditory interactions in sensorimotor processing: saccades versus manual responses. J Exp Psychol Hum Percept Perform 20: 131–153
Hughes HC, Nelson MD, Aronchick DM (1998) Spatial characteristics of visual–auditory summation in human saccades. Vis Res 38:3955–3963
Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE (1997) Mechanisms of within- and cross-modality suppression in the superior colliculus. J Neurophysiol 78:2834–2847
King AJ, Palmer AR (1985) Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp Brain Res 60:492–500
Lewald J, Guski R (2003) Cross-modal perceptual integration of spatially and temporally disparate auditory and visual stimuli. Cogn Brain Res 16:468–478
Lorenzi C, Gatehouse S, Lever C (1999) Sound localization in normal-hearing listeners. J Acoust Soc Am 99:1810–1820
Lueck CJ, Crawford TJ, Savage CJ, Kennard C (1990) Auditory–visual interaction in the generation of saccades in man. Exp Brain Res 82:149–157
Ma WJ, Pouget A (2008) Linking neurons to behavior in multisensory perception: a computaitonal review. Brain Res 1242:4–12
McIlwain JT (1986) Effect of eye position on saccades evoked electrically from superior colliculus of alert cats. J Neurophysiol 55:97–112
Meredith MA, Nemitz JW, Stein BE (1987) Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci 10:3215–3229
Meredith MA, Stein BE (1986) Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56:640–662
Meredith MA, Stein BE (1996) Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol 75:1843–1857
Middlebrooks JC, Knudsen EI (1984) A neural code for auditory space in the cat’s superior colliculus. J Neurosci 4:2621–2634
Miller J (1982) Divided attention: evidence for coactivation with redundant signals. Cogn Psychol 14:247–279
Morein-Zamir S, Soto-Faraco S, Kingstone A (2003) Auditory capture of vision: examining temporal ventriloquism. Cogn Brain Res 17:154–163
Munoz DP, Fecteau JH (2002) Vying for dominance: dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Prog Brain Res 140:3–19
Munoz DP, Schall JD (2004) Concurrent, distributed control of saccade initiation in the frontal eye field and superior colliculus. In: Hall WC, Moschovakis A (eds) The superior colliculus: new approaches for studying sensorimotor integration. CRC Press, Boca Raton, pp 55–82
Populin LC, Yin TC (2002) Bimodal interactions in the superior colliculus of the behaving cat. J Neurosci 22:2826–2834
Raab DH (1962) Statistical facilitation of simple reaction times. Trans N Y Acad Sci 24:574–590
Rowland BA, Quessy S, Stanford TR, Stein BE (2007) Multisensory integration shortens physiological response latencies. J Neurosci 27(22):5879–5884
Rowland BA, Stein BE (2007) Multisensory integration produces an initial response enhancement. Front Integr Neurosci 1:4. doi:10.3389/neuro.07.004.2007
Sparks DL (1999) Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9:698–707
Sparks DL, Freedman EG, Chen LL, Gandhi NJ (2001) Cortical and subcortical contributions to coordinated eye and head movements. Vis Res 41:3295–3305
Spence C, Squire S (2003) Multisensory integration: maintaining the perception of synchrony. Curr Biol 13:R519–R521
Steenken R, Colonius H, Diederich A, Rach S (2008) Visual–auditory interaction in saccadic reaction time: effects of auditory masker level. Brain Res 1220:150–156
Stein BE (1998) Neural mechanisms for synthesizing sensory information and producing adaptive behavior. Exp Brain Res 123: 124–135
Stein BE, Meredith MA (1993) The merging of the senses. The MIT Press, Cambridge
van Atteveldt NM, Formisano E, Blomert L, Goebel R (2007) The effect of temporal asynchrony on the multisensory integration of letters and speech sounds. Cereb Cortex 17(4):962–974
Van Opstal AJ, Munoz DP (2004) Auditory–visual interactions subserving primate gaze orienting. In: Calvert G, Spence C, Stein BE (eds) Handbook of multisensory processes, Cambridge, MIT Press, pp 373–393
Wallace MT, Wilkinson LK, Stein BE (1996) Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol 76:1246–1266
Whitchurch EA, Takahashi TT (2006) Combined auditory and visual stimuli facilitate head saccades in the barn owl (Tyto alba). J Neurophysiol 96:730–745
Wightman FL, Kistler DJ (1989) Headphone simulation of free field listening I: stimulus synthesis. J Acoust Soc Am 85:858–867
Acknowledgment
This research was supported by Grants from Deutsche Forschungsgemeinschaft Di 506/8-1 and Di 506/8/-3 and SFB-TR31 (Active Listening).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Multisensory Integration.
Appendix
Appendix
The race in the first stage of the model is made explicit by assigning independent non-negative random variables V and A to the peripheral processing times for the visual target and auditory non-target stimulus, respectively. With τ as SOA value and ω as integration window width parameter, the time window of integration assumption is equivalent to the (stochastic) event I, say,
Thus, the probability of integration to occur, P(I), is a function of both τ and ω, and it can be determined numerically once the distribution functions of A and V have been specified.
The next step is to compute expected reaction time for the unimodal and crossmodal conditions. From the two-stage assumption, total reaction time in the crossmodal condition can be written as a sum of two random variables:
where S 1 and S 2 refer to the first and second stage processing time, respectively. For the expected saccadic reaction time in the crossmodal condition then follows:
where E[S 2|I] and E[S 2|not-I] denote the expected second stage processing time conditioned on interaction occurring (I) or not occurring (not-I), respectively. Setting
this becomes
In the unimodal condition, no integration is possible. Thus,
and we arrive at the simple product rule for expected crossmodal interaction (ECI)
Parameters were estimated by minimizing the Pearson χ2 statistic
using the FMINSEARCH routine of MATLAB. Here \(\overline{SRT}(j,n)\) and \(\widehat{SRT}(j,n)\) are, respectively, the observed and the fitted values of the mean SRT to visual–auditory stimuli) presented in spatial positions (coincident, j = 1; disparate, j = 2) with SOA (referred to by n = 1 to 4); \(\sigma_{\overline{SRT}_{(j,n)}}\) are the respective standard errors.
For a more detailed formal presentation of the model we refer to Diederich and Colonius (2008a).
Rights and permissions
About this article
Cite this article
Colonius, H., Diederich, A. & Steenken, R. Time-Window-of-Integration (TWIN) Model for Saccadic Reaction Time: Effect of Auditory Masker Level on Visual–Auditory Spatial Interaction in Elevation. Brain Topogr 21, 177–184 (2009). https://doi.org/10.1007/s10548-009-0091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-009-0091-8