Abstract
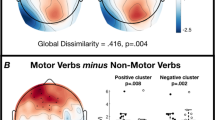
Previous neuroanatomical research has shown that semantic processing of action-related language activates the premotor, motor, and sensory cortices somatotopically (e.g., Tettamanti et al., J Cognitive Neurosci. 2005;17(2): 273–281, using a listening task, and Hauk et al., Neuron. 2004;41:301–307 and Pulvermuller et al., Eur J Neurosci 2005;21:793–797; J Cognitive Neurosci 2005;17(6):884–892 using a silent reading task). We examined this somatotopic semantics hypothesis using an overt semantic generation task (i.e., participants generated aloud their own personal description of how they would interact with target object words), rather than semantic comprehension as examined in previous research, so as to provide a stronger test of the hypothesis under conditions that tap one’s own semantic knowledge about interacting with objects. Experiment 1 used functional Magnetic Resonance Imaging (fMRI) to examine somatotopically organized activation in the premotor cortex for an overt semantic generation task, using targets that naturally involve either arm interactions or leg interactions. Consistent with previous research, our results showed that semantic processing related to object interaction involves the motor, premotor and sensory cortices in a somatotopic fashion. Previous behavioural research has shown a response advantage in lexical decision for words with multiple meanings or features, which diminishes with tasks that decrease semantic involvement (e.g., Borowsky and Masson, J Exp Psychol: Learn Memory Cognit 1996; 22(1):63–85; Pexman et al., Psychon Bull Rev 2002;9(3): 542–549). Experiment 2 evaluated whether semantic generation response times (total duration of response) display a complexity advantage (i.e., faster response times for more complex objects), and whether complexity ratings were related to the volume of brain activation during the task. Results from this behavioural experiment revealed a significant negative relationship between the total duration of response (i.e., the total amount of time taken to respond to the stimuli) and object complexity for leg objects (a semantic complexity advantage), but not for arms. This suggests that the smaller repertoire of possible interactions with leg objects requires a greater reliance on semantic knowledge in order to respond in the semantic generation task. This interpretation was further supported by a greater volume of brain activation in the premotor cortex for arm objects versus leg objects. The response times from Experiment 1 were also compared to the semantic complexity ratings gathered in Experiment 2 to determine if response times in the fMRI environment were affected by how complex an object is.
Similar content being viewed by others
References
Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H. Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci. 2007;27(13):3429–38.
Allport DA. Distributed memory, modular subsystems, and dysphasia. In: Newman SK, Epstein R, editors. Current perspectives in dysphasia. Edinburgh: Churchill, Livingstone; 1985. p. 207–44.
Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999; 22:577–660.
Borowsky R, Cummine J, Owen WJ, Friesen CK, Shih F, Sarty GE. FMRI of ventral and dorsal processing streams in basic reading processes: insular sensitivity to phonology. Brain Topogr. 2006;18(4):233–9.
Borowsky R, Loehr J, Friesen CK, Kraushaar G, Kingstone A, Sarty G. Modularity and intersection of ‹what’, ‹where’, and ‹how’ processing of visual stimuli: a new method of fMRI localization. Brain Topogr. 2005;18:67–75.
Borowsky R, Owen WJ, Sarty GE. The role of the left hemisphere in motor control of touch: a functional magnetic resonance imaging analysis. Brain Cognition. 2002;49:96–101.
Borowsky R, Masson MEJ. Semantic ambiguity effects in word identification. J Exp Psychol: Learn Mem Cogn. 1996;22(1):63–85.
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13: 400–4.
Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–84.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29: 162–173. [AFNI 3-d anatomical brain available at:http://afni.nimh.nih.gov/old/afni/astrip+orig.HEAD (and BRIK).
Gerardin E, Sirigu A, Lehericy S, Poline J, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10:1093–104.
Kan IP, Kable JW, Van Scoyoc A, Chatterjee A, Thompson-Schill SL. Fractioning the left frontal response to tools: dissociable effects of motor experience and lexical competition. J Cognitive Neurosci. 2006;18(2):267–77.
Hauk O, Pulvermuller F. Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Map. 2004;21: 191–201.
Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004; 41:301–7.
Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol. 1999;9:735–9.
Martin A. The representation of object concepts in the brain. Ann Rev Psychol. 2007;58:25–45.
Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201.
Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270(5233):102–8.
Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–52.
Owen WJ, Borowsky R, Sarty GE. FMRI of two measures of phonological processing in visual word recognition: ecological validity matters. Brain Lang. 2004;90:40–6.
Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1958;60:389–443.
Pexman PM, Lupker SJ, Hino Y. The impact of feedback semantics in visual word recognition: number-of-features effects in lexical decision and naming tasks. Psychon Bull Rev. 2002;9(3): 542–9.
Pexman PM, Hino Y, Lupker SJ. Semantic ambiguity and the process of generating meaning from print. J Exp Psychol: Learn Mem Cogn. 2004;30:1252–70.
Pulvermuller F. Brain mechanisms linking language and action. Nat Rev. 2005;6:576–81.
Pulvermuller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. EurJ Neurosci. 2005a;21: 793–7.
Pulvermuller F, Shtyrov Y, Ilmoniemi RJ. Brain signatures of meaning access in action word recognition. J Cognitive Neurosci. 2005b;17(6):884–92.
Sarty GE. Computing brain activity maps from fMRI time-series images. New York: Cambridge University Press; 2007.
Sarty G, Borowsky R. Functional MRI activation maps from empirically defined curve fitting. Magn Reson Eng. 2005;24b:46–55.
Siakaluk PD, Pexman PM, Sears CR, Wilson K, Locheed K, Owen WJ. The benefits of sensorimotor knowledge: body-object interaction facilitates semantic processing. Cognitive Sci. (in press).
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc.;1988.
Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, et al. Listening to action-related sentences activates fronto-parietal motor circuits. J Cognitive Neurosci. 2005;17(2):273–81.
Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia. 2003;41:280–92.
Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization in memory. New York: Academic Press; 1972. p. 381–403.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada in the form of grants to R.B. and G.E.S. R.B.’s contribution is equivalent to first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esopenko, C., Borowsky, R., Cummine, J. et al. Mapping the Semantic Homunculus: A Functional and Behavioural Analysis of Overt Semantic Generation. Brain Topogr 21, 22–35 (2008). https://doi.org/10.1007/s10548-008-0043-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-008-0043-8