Abstract
Background
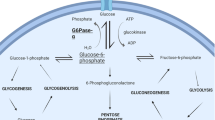
Viral mediated gene therapy has progressed after overcoming early failures, and gene therapy has now been approved for several conditions in Europe and the USA. Glycogen storage disease (GSD) type Ia, caused by a deficiency of glucose-6-phosphatase-α, has been viewed as an outstanding candidate for gene therapy. This follow-up report describes the long-term outcome for the naturally occurring GSD-Ia dogs treated with rAAV-GPE-hG6PC-mediated gene therapy.
Methods
A total of seven dogs were treated with rAAV-GPE-hG6PC-mediated gene therapy. The first four dogs were treated at birth, and three dogs were treated between 2 and 6 months of age to assess the efficacy and safety in animals with mature livers. Blood and urine samples, radiographic studies, histological evaluation, and biodistribution were assessed.
Results
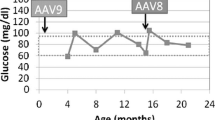
Gene therapy improved survival in the GSD-Ia dogs. With treatment, the biochemical studies normalized for the duration of the study (up to 7 years). None of the rAAV-GPE-hG6PC-treated dogs had focal hepatic lesions or renal abnormalities. Dogs treated at birth required a second dose of rAAV after 2–4 months; gene therapy after hepatic maturation resulted in improved efficacy after a single dose.
Conclusion
rAAV-GPE-hG6PC treatment in GSD-Ia dogs was found to be safe and efficacious. GSD-Ia is an attractive target for human gene therapy since it is a monogenic disorder with limited tissue involvement. Blood glucose and lactate monitoring can be used to assess effectiveness and as a biomarker of success. GSD-Ia can also serve as a model for other hepatic monogenic disorders.



Similar content being viewed by others
References
Brix AE, Howerth EW, McConkie-Rosell A et al (1995) Glycogen storage disease type Ia in two littermate Maltese puppies. Vet Pathol 32(5):460
Chen MA, Weinstein DA (2016) Glycogen storage diseases: diagnosis, treatment and outcome. Transl Sci Rare Dis 1:45–72
Chou JY, Mansfield BC (2011) Recombinant AAV-directed gene therapy for type I glycogen storage diseases. Expert Opin Biol Ther 11:1011–1024
Chou JY, Matern D, Mansfield BC, Chen YT (2002) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med 2:121–143
Chou JY, Jun HS, Mansfield BC (2010) Glycogen storage disease type I and G6Pase-beta deficiency: etiology and therapy. Nat Rev Endocrinol 6:676–688
Cideciyan AV, Hauswirth WW, Aleman TS et al (2009) Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 361:725–727
Clar J, Gri B, Calderaro J et al (2014) Targeted deletion of kidney glucose-6 phosphatase leads to nephropathy. Kidney Int 86:747–756
Cramer ML, Shao G, Rodino-Klapac LR, Chicoine LG, Martin PT (2017) Induction of t-cell infiltration and programmed death ligand 2 expression by adeno-associated virus in rhesus macaque skeletal muscle and modulation by prednisone. Hum Gene Ther 28:493–509
Cunningham SC, Dane AP, Spinoulas A, Logan GJ, Alexander IE (2008) Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther 16:1081–1088
Dambska M, Labrador EB, Kuo CL, Weinstein DA (2017) Prevention of complications in glycogen storage disease type Ia with optimization of metabolic control. Pediatr Diabetes 18:327–331
Davis MK, Weinstein DA (2008) Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant 12:137–145
Demaster A, Luo X, Curtis S et al (2012) Long-term efficacy following readministration of an adeno-associated virus vector in dogs with glycogen storage disease type Ia. Hum Gene Ther 23:407–418
Gaudet D, de Wal J, Tremblay K et al (2010) Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl 11(1):55–60
Ghosh A, Allamarvdasht M, Pan C-J et al (2006) Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther 13:321–329
Hacein-Bey-Abina S, Le Deist F, Carlier F et al (2002) Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 346:1185–1193
Hacein-Bey-Abina S, von Kalle C, Schmidt M et al (2003) A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 348:255–256
Kim GY, Lee YM, Kwon JH et al (2017) Glycogen storage disease type Ia mice with less than 2% of normal hepatic glucose-6-phosphatase-α activity restored are at risk of developing hepatic tumors. Mol Genet Metab 120:229–234
Kishnani PS, Bao Y, Wu JY, Brix AE, Lin JL, Chen YT (1997) Isolation and nucleotide sequence of canine glucose-6-phosphatase mRNA: identification of mutation in puppies with glycogen storage disease type Ia. Biochem Mol Med 61:168–177
Koeberl DD, Pinto C, Sun B et al (2008) AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol Ther 16:665–672
Lee YM, Jun HS, Pan CJ et al (2012) Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology 56:1719–1729
Lee YM, Pan CJ, Koeberl DD, Mansfield BC, Chou JY (2013) The upstream enhancer elements of the G6PC promoter are critical for optimal G6PC expression in murine glycogen storage disease type Ia. Mol Genet Metab 110:275–280
Lei KJ, Chen H, Pan CJ et al (1996) Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type 1a mouse. Nat Genet 13:203–209
Maguire AM, Simonelli F, Pierce EA et al (2008) Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248
Mutel E, Abdul-Wahed A, Ramamonjisoa N et al (2011) Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J Hepatol 54:529–537
Nathwani AC, Reiss UM, Tuddenham EG et al (2014) Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 371:1994–2004
Okechuku GO, Shoemaker LR, Dambska M, Brown LM, Mathew J, Weinstein DA (2017) Tight metabolic control plus ACE inhibitor therapy improves GSD I nephropathy. J Inherit Metab Dis 40:703–708
Rajas F, Clar J, Gautier-Stein A, Mithieux G (2015) Lessons from new mouse models of glycogen storage disease type Ia in relation to the time course and organ specificity of the disease. J Inherit Metab Dis 38:521–527
Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP (2002) Glycogen storage disease type I: diagnosis, management, clinical course, and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr 161(Suppl 1):S20–S34
Rangarajan S, Walsh L, Lester W (2017) AAV5–factor VIII gene transfer in severe hemophilia a. N Engl J Med 377:2519–2530
Reddy SK, Austin SL, Spencer-Manzon M et al (2009) Liver transplantation for glycogen storage disease type Ia. J Hepatol 51:483–490
Rogers S, Pfuderer P (1968) Use of viruses as carriers of added genetic information. Nature 219:749–751
Rogers S, Lowenthal A, Terheggen HG, Columbo JP (1973) Induction of arginase activity with the Shope papilloma virus in tissue culture cells from an argininemic patient. J Exp Med 137:1091–1096
Sambrook J, Westphal H, Srinivasan PR, Dulbecco R (1968) The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A 60:1288–1295
Simonelli F, Maguire AM, Testa F et al (2010) Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18:643–650
Specht A, Fiske L, Erger K et al (2011) Glycogen storage disease type Ia in canines: a model for human metabolic and genetic liver disease. J Biomed Biotechnol 2011:646257
Stolberg SG (1999) The biotech death of Jesse Gelsinger. N Y Times Mag 136-140:149–150
Tatum EL (1966) Molecular biology, nucleic acids, and the future of medicine. Perspect Biol Med 10:19–32
Temin HM (1961) Mixed infection with two types of Rous sarcoma virus. Virology 13:158–163
Wang DQ, Fiske LM, Carreras CT, Weinstein DA (2011) Natural history of hepatocellular adenoma formation in glycogen storage disease type I. J Pediatr 159:442–446
Weinstein DA, Wolfsdorf JI (2002) Effect of continuous glucose therapy with uncooked cornstarch on the long-term clinical course of type 1a glycogen storage disease. Eur J Pediatr Suppl 1:S35–S39
Weinstein DA, Correia CE, Conlon T et al (2010) Adeno-associated virus-mediated correction of a canine model of glycogen storage disease type Ia. Hum Gene Ther 21:903–910
Yiu WH, Lee YM, Peng WT et al (2010) Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol Ther 18:1076–1084
Ylä-Herttuala S (2012) Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol Ther 20:1831–1832
Zolotukhin S, Potter M, Zolotukhin I et al (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28:158–167
Acknowledgements
The authors gratefully acknowledge the University of Florida GSD Puppy Care Team and the University of Florida Animal Care Services and College of Veterinary Medicine Veterinary Staff for their assistance in animal care. The authors also thank and acknowledge Dr. Catherine Mah. Dr. John Verstegen, Dr. Maggie Struck, Ms. Catherine Correia, Dr. Barry Byrne and Mr. Travis Cossette for their efforts to get this work started. Mr. Noah Drazen is recognized for his assistance in preparing this manuscript.
Funding
This research was made possible grant support provided by the Global Center for GSD, the Children’s Fund for GSD Research, Children’s Miracle Network, and the National Institutes of Health (NHLBI P01 HL59412-06 and NIDDK P01 DK58327-03). Philanthropic support was also proved by the Fry Family Foundation and the following funds managed through the University of Florida Office of Development: Scott Miller GSD Program Fund, Matthew Ehrman GSD Research Fund, Jonah Pournazarian GSD Ib Research Fund, GSD Dream Fund, Green Family Fund for GSD Research, Canadian Fund for the Cure of GSD, Team Tallulah for Type Ib Research, and Jamie Konieczka Type Ib GSD Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
These investigations were approved by and performed in accordance with the guidelines of the University of Florida Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicted by: Terry Derks
Young Mok Lee and Thomas J. Conlon served as co-primary investigators for this work
Electronic supplementary material
Supplement figure 1
Biochemical Characteristics Over Time (additional values). Serum analysis for Total Biliruvin, Total Protein, Albumin, Globulin, Calcium, Phosphorus, Magnesium, Sodium, Potassium, Chloride, Total CO2 and Anion Gap. Black line with round symbol (CO), Red line with square symbol (GE), Blue line with triangle (TU) and Purple line with reversed triangle (JA). Area between dashed line indicates normal ranges of the enzymes or blood metabolites (DOCX 168 kb)
Rights and permissions
About this article
Cite this article
Lee, Y.M., Conlon, T.J., Specht, A. et al. Long-term safety and efficacy of AAV gene therapy in the canine model of glycogen storage disease type Ia. J Inherit Metab Dis 41, 977–984 (2018). https://doi.org/10.1007/s10545-018-0199-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0199-7