Abstract
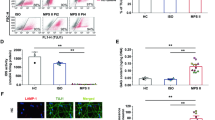
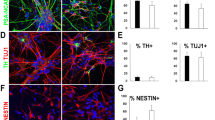
Mucopolysaccharidosis type II (MPSII) is a rare X-linked lysosomal storage disorder caused by mutations in the iduronate-2-sulfatase (IDS) gene (IDS, Xq28). MPSII is characterized by skeletal deformities, hearing loss, airway obstruction, hepatosplenomegaly, cardiac valvular disease, and progressive neurological impairment. At the cellular level, IDS deficiency leads to lysosomal storage of glycosaminoglycans (GAGs), dominated by accumulation of dermatan and heparan sulfates. Human induced pluripotent stem cells (iPSC) represent an alternative system that complements the available MPSII murine model. Herein we report on the reprogramming of peripheral white blood cells from male and female MPSII patients into iPSC using a non-integrating protocol based on the Sendai virus vector system. We differentiated the iPSC lines into IDS deficient and GAG accumulating β-Tubulin III+ neurons, GFAP+ astrocytes, and CNPase+ oligodendrocytes. The lysosomal system in these cells displayed structural abnormalities reminiscent of those previously found in patient tissues and murine IDS deficient neuronal stem cells. Furthermore, quantitative determination of GAGs revealed a moderate increase in GAG levels in IDS deficient neurons and glia. We also tested the effects of recombinant IDS and found that the exogenous enzyme was internalized from the culture media and partially decreased the intracellular GAG levels in iPSC-derived neural cells; however, it failed to completely prevent accumulation of GAGs. In summary, we demonstrate that this human iPSC based model expresses the cellular and biochemical features of MPSII, and thus represents a useful experimental tool for further pathogenesis studies as well as therapy development and testing.




Similar content being viewed by others
Abbreviations
- ALP:
-
alkaline phosphatase
- BBB:
-
blood brain barrier
- bFGF:
-
basic fibroblast growth factor
- βTubIII:
-
β-Tubulin III
- CNPase:
-
2′,3′-cyclic-nucleotide 3′-phosphodiesterase
- EB:
-
embryoid bodies
- ERT:
-
enzyme replacement therapy
- FGF-8:
-
fibroblast growth factor 8
- GAG:
-
glycosaminoglycans
- GFAP:
-
glial fibrillary acidic protein
- HS:
-
heparan sulfate
- IDS:
-
iduronate-2-sulfatase
- iPSC:
-
induced pluripotent stem cells
- LAMP1:
-
lysosomal-associated membrane protein 1
- MAP2:
-
microtubule-associated protein 2
- MPS:
-
mucopolysaccharidoses
- NPC:
-
neural progenitor cells
- PBMC:
-
peripheral blood mononuclear cells
- P/S:
-
Penicillin-Streptomycin
- TH:
-
tyrosine hydroxylase
- XCI:
-
X-chromosome inactivation
- CDM:
-
complete differentiation medium
References
All AH, Gharibani P, Gupta S et al (2015) Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One 10:e0116933
Boado RJ, Hui EK, Lu JZ, Sumbria RK, Pardridge WM (2013) Blood-brain barrier molecular trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey. Bioconjug Chem 24:1741–1749
Canfield SG, Stebbins MJ, Morales BS et al (2017) An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem 140:874–888
Cardone M, Polito VA, Pepe S et al (2006) Correction of hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum Mol Genet 15:1225–1236
Chen J, Riazifar H, Guan MX, Huang T (2016) Modeling autosomal dominant optic atrophy using induced pluripotent stem cells and identifying potential therapeutic targets. Stem Cell Res Ther 7:2
Fusar Poli E, Zalfa C, D'Avanzo F et al (2013) Murine neural stem cells model hunter disease in vitro: glial cell-mediated neurodegeneration as a possible mechanism involved. Cell Death Dis 4:e906
Gallagher JT (2006) Multiprotein signalling complexes: regional assembly on heparan sulphate. Biochem Soc Trans 34:438–441
Gomes IC, Acquarone M, Maciel Rde M, Erlich RB, Rehen SK (2010) Analysis of pluripotent stem cells by using cryosections of embryoid bodies. J Vis Exp. doi: 10.3791/2344
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Itier JM, Ret G, Viale S et al (2014) Effective clearance of GL-3 in a human iPSC-derived cardiomyocyte model of Fabry disease. J Inherit Metab Dis 37:1013–1022
Jakobkiewicz-Banecka J, Gabig-Ciminska M, Kloska A et al (2016) Glycosaminoglycans and mucopolysaccharidosis type III. Front Biosci (Landmark Ed) 21:1393–1409
Jones SA, Parini R, Harmatz P, Giugliani R, Fang J, Mendelsohn NJ (2013) The effect of idursulfase on growth in patients with hunter syndrome: data from the hunter outcome survey (HOS). Mol Genet Metab 109:41–48
de Jong JG, Wevers RA, Liebrand-van Sambeek R (1992) Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem 38:803–807
Meier C, Wismann U, Herschkowitz N, Bischoff A (1979) Morphological observations in the nervous system of prenatal mucopolysaccharidosis II (M. Hunter). Acta Neuropathol 48:139–143
Meng XL, Shen JS, Kawagoe S, Ohashi T, Brady RO, Eto Y (2010) Induced pluripotent stem cells derived from mouse models of lysosomal storage disorders. Proc Natl Acad Sci U S A 107:7886–7891
Motas S, Haurigot V, Garcia M et al (2016) CNS-directed gene therapy for the treatment of neurologic and somatic mucopolysaccharidosis type II (hunter syndrome). JCI Insight 1:e86696
Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA (2002) Enzyme replacement therapy in mucopolysaccharidosis type II (hunter syndrome): a preliminary report. Acta Paediatr Suppl 91:98–99
Muenzer J, Beck M, Eng CM et al (2011) Long-term, open-labeled extension study of idursulfase in the treatment of hunter syndrome. Gen Med: Off J Am Coll Med Gen 13:95–101
Neufeld EF, Muenzer J (2001) The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS (eds) The metabolic and molecular basis of inherited disease. McGraw-Hill, New York, pp 3421–3452
Noh H, Lee JI (2014) Current and potential therapeutic strategies for mucopolysaccharidoses. J Clin Pharm Ther 39:215–224
Okuyama T, Tanaka A, Suzuki Y et al (2010) Japan Elaprase treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated hunter syndrome (mucopolysaccharidosis II, MPS II). Mol Genet Metab 99:18–25
Patel P, Suzuki Y, Tanaka A et al (2014) Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with hunter syndrome. Mol Gen Metab Rep 1:184–196
Perrimon N, Bernfield M (2001) Cellular functions of proteoglycans--an overview. Semin Cell Dev Biol 12:65–67
Polito VA, Cosma MP (2009) IDS crossing of the blood-brain barrier corrects CNS defects in MPSII mice. Am J Hum Genet 85:296–301
Polito VA, Abbondante S, Polishchuk RS, Nusco E, Salvia R, Cosma MP (2010) Correction of CNS defects in the MPSII mouse model via systemic enzyme replacement therapy. Hum Mol Genet 19:4871–4885
Russo FB, Cugola FR, Fernandes IR, Pignatari GC, Beltrao-Braga PC (2015) Induced pluripotent stem cells for modeling neurological disorders. World J Transplant 5:209–221
Sarrazin S, Lamanna WC, Esko JD (2011) Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3:a004952
Skoog WA, Beck WS (1956) Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood 11:436–454
Stacpoole SR, Bilican B, Webber DJ et al (2011) Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen. Nat Protoc 6:1229–1240
Takahashi K, Yamanaka S, (2006) Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126 (4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Tanaka A, Okuyama T, Suzuki Y et al (2012) Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab 107:513–520
Varki A (1999) Essentials of glycobiology, vol xvii. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, p 653
Voznyi YV, Keulemans JL, van Diggelen OP (2001) A fluorimetric enzyme assay for the diagnosis of MPS II (hunter disease). J Inherit Metab Dis 24:675–680
Wiesmann UN, Spycher MA, Meier C, Liebaers I, Herschkowitz N (1980) Prenatal mucopolysaccharidosis II (hunter): a pathogenetic study. Pediatr Res 14:749–756
Wraith JE, Scarpa M, Beck M et al (2008) Mucopolysaccharidosis type II (hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr 167:267–277
Acknowledgments
The authors thank Irena Knesplova and Dr. Filip Majer for their technical assistance and Dr. Martin Magner for assistance with collection of patient samples and clinical evaluation.
Funding
This work was funded by a research grant of the Medical Research Agency of The Czech Republic, AZV ČR 15-33297A.
The authors confirm independence from the sponsors; the content of the article was not influenced by the sponsors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
Jitka Rybova, Robert Dobrovolny, Jana Ledvinova, Jakub Sikora and Ladislav Kuchar declare no competing interests but disclose the following: Robert Dobrovolny and Jitka Rybova have received honoraria for lectures and/or hotel/travel expenses for relevant meetings from Shire, plc.
Animal rights
This article does not contain any studies with animal subjects performed by any of the authors.
Conflict of interest
J. Rybova, J. Ledvinova, J. Sikora, L. Kuchar, R. Dobrovolny declare that they have no conflict of interest.
Additional information
Communicated by: Jaak Jaeken
Electronic supplementary material
Figure S1
(GIF 241 kb)
Fig. S2
(PDF 497 kb)
Table S1
(DOCX 16 kb)
Supplemental Materials and methods
(DOCX 36.6 kb)
Rights and permissions
About this article
Cite this article
Rybová, J., Ledvinová, J., Sikora, J. et al. Neural cells generated from human induced pluripotent stem cells as a model of CNS involvement in mucopolysaccharidosis type II. J Inherit Metab Dis 41, 221–229 (2018). https://doi.org/10.1007/s10545-017-0108-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0108-5