Abstract
Objectives
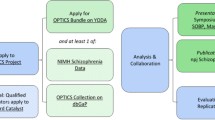
The common data elements (CDE) project was developed by the National Institute of Neurological Disorders and Stroke (NINDS) to provide clinical researchers with tools to improve data quality and allow for harmonization of data collected in different research studies. CDEs have been created for several neurological diseases; the aim of this project was to develop CDEs specifically curated for mitochondrial disease (Mito) to enhance clinical research.
Methods
Nine working groups (WGs), composed of international mitochondrial disease experts, provided recommendations for Mito clinical research. They initially reviewed existing NINDS CDEs and instruments, and developed new data elements or instruments when needed. Recommendations were organized, internally reviewed by the Mito WGs, and posted online for external public comment for a period of eight weeks. The final version was again reviewed by all WGs and the NINDS CDE team prior to posting for public use.
Results
The NINDS Mito CDEs and supporting documents are publicly available on the NINDS CDE website (https://commondataelements.ninds.nih.gov/), organized into domain categories such as Participant/Subject Characteristics, Assessments, and Examinations.
Conclusion
We developed a comprehensive set of CDE recommendations, data definitions, case report forms (CRFs), and guidelines for use in Mito clinical research. The widespread use of CDEs is intended to enhance Mito clinical research endeavors, including natural history studies, clinical trial design, and data sharing. Ongoing international collaboration will facilitate regular review, updates and online publication of Mito CDEs, and support improved consistency of data collection and reporting.

Similar content being viewed by others
Change history
04 October 2017
An erratum to this article has been published.
References
Camp KM, Krotoski D, Parisi MA, Gwinn KA, Cohen BH, Cox CS, Enns GM, Falk MJ, Goldstein AC, Gopal-Srivastava R, Gorman GS, Hersh SP, Hirano M, Hoffman FA, Karaa A, MacLeod EL, McFarland R, Mohan C, Mulberg AE, Odenkirchen JC, Parikh S, Rutherford PJ, Suggs-Anderson SK, Tang WH, Vockley J, Wolfe LA, Yannicelli S, Yeske PE, Coates PM (2016) Nutritional interventions in primary mitochondrial disorders: developing an evidence base. Mol Genet Metab 119(3):187–206
Chinnery PF (2014) Mitochondrial disorders overview. In: Pagon PA, Adam MP, Ardinger HH et al (eds) Gene reviews. University of Washington, Seattle
DiMauro S, Schon E (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668
Gorman GS, Schaefer AM, Ng Y et al (2015) Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 77:753–759
Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM (2016) Mitochondrial diseases. Nat Rev Dis Primers. doi:10.1038/nrdp.2016.80
Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S (2012) National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clin Trials 9:322–329
Harrison SM, Riggs ER, Maglott DR, Lee JM, Azzariti DR, Niehaus A, Ramos EM, Martin CL, Landrum MJ, Rehm HL (2016) Using ClinVar as a resource to support variant interpretation. Curr Protoc Hum Genet 89:8.16.1–8.16.23
Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V, Wallace DC (2013) mtDNA variation and analysis using mitomap and mitomaster. Curr Protoc Bioinformatics 44:1.23.1–26
MacArthur DG, Manolio TA, Dimmock DP (2014) Guidelines for investigating causality of sequence variants in human disease. Nature 24 508(7497):469–476
Nightingale H, Pfeffer G, Bargiela D, Horvath R, Chinnery PF (2016) Emerging therapies for mitochondrial disorders. Brain 139:1633–1648
Niyazov D, Kahler S, Frye R (2016) Primary mitochondrial disease and secondary mitochondrial dysfunction: importance of distinction for diagnosis and treatment. Mol Syndromol 7:122–137
Parikh S, Goldstein A, Koenig MK et al (2015) Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med 17:689–701
Rahman S (2015) Emerging aspects of treatment in mitochondrial disorders. J Inherit Metab Dis 38:641–653
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Suomalainen A (2013) Fibroblast growth factor 21: a novel biomarker for human muscle-manifesting mitochondrial disorders. Expert Opin Med Diagn 7:313–317
Yatsuga S, Fujita Y, Ishii A et al (2015) Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol 7:814–823
Acknowledgements
The views expressed here are those of the authors and do not represent those of the NIH, NINDS or the US Government. Logistic support for this project was provided in part through NIH Contract HHSN271201200034C. The development of the NINDS Mito CDEs was made possible thanks to the great investment of time and effort of WG members and the members of the NINDS CDE Project team participating from 2013 to 2015.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
John Shoffner has received research funds from the Department of Defense and owns stock in Medical Neurogenetics, LLC.
David Dimmock is a consultant to Audentes Therapeutics, Biomarin, Ilumina and Complete Genomics and involved in clinical trials with Genzyme/Sanofi, Biomarin, Shire, Cytonet.
Marni Falk has been a consultant to Mitokyne; received research funds from Cardero Therapeutics, Mitobridge, Raptor Pharmaceuticals, RiboNova, Stealth BioTherapeutics, and Vitaflo International; was a former scientific advisory board member for Perlstein Laboratory, Inc., and is involved in clinical trials with REATA pharmaceuticals.
Amel Karaa, Shamima Rahman, Anne Lombès, Patrick Yu-Wai-Man, Muniza K. Sheikh, Sherita Alai-Hansen, Bruce H. Cohen, Lisa Emrick, Shana McCormack, David Mirsky, Tony Moore, Sumit Parikh, Tanja Taivassalo, Mark Tarnopolsky, Ingrid Tein, Joanne C. Odenkirchen, and Amy Goldstein have no conflict of interest.
Studies with human or animal
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Additional information
Communicated by: Eva Morava
MITO Working Group Members
Biomarkers WG
John Shoffner, MD (Chair), Atlanta, GA, USA
Mark Bamberger, PhD, Stealth Peptides, Inc, Newton Centre, MA, USA
Yasutoshi Koga, MD, PhD, Kurume University, Fukuoka-ken, Japan
Anne Lombès, MD, PhD, INSERM, Paris, France
Fernando Scaglia, MD, Baylor College of Medicine, Houston, TX, USA
Cognitive/Behavioral/Psychological Outcomes WG
Lisa Emrick, MD (Chair), Baylor College of Medicine/Texans Children’s Hospital, Houston, TX, USA
Rebecca Vaurio, PhD, Kennedy Krieger Institute, Baltimore, MD, USA
Endocrinology/Diabetes/GI/Nutrition WG
Shana McCormack, MD (Chair), The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
J.E. Abdenur, MD, CHOC Children’s Hospital, Orange, CA, USA
Kristin Fiorino, MD, Children’s Hospital of Philadelphia, Philadelphia, PA, USA (ad hoc member)
Bret Goodpaster, PhD, Sanford, Burnham Medical Research Institute, Orlando, FL, USA
Robert O. Heuckeroth, MD PhD, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Kierstin Keller, CGC, Children’s Hospital of Philadelphia, Philadelphia, PA, USA (ad hoc member)
Robert McFarland, DoH, HEFCE, Newcastle University, UK
Philip Morgan, MD, Mitochondrial Research Guild, Seattle, WA, USA
Fernando Scaglia, MD, Baylor College of Medicine, Houston, TX, USA
Charles A. Stanley, MD, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Steven M. Willi, MD, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Exercise Physiology WG
Tanja Taivassalo, PhD (Chair), McGill University, Montreal, QC, Canada
Amy Goldstein, MD, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Ronald Haller, MD, Southwestern Medical Center, Dallas, TX, USA
Mark Tarnopolsky, MD, PhD, McMaster University, Hamilton, Ontario, Canada
Karim Wahbi, MD, PhD, Institute of Myology, Paris, France
Genetics WG
David Dimmock, MD (Co-Chair), Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Marn J. Falk, MD (Co-Chair), The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Xiaowu Gai, PhD, Ocular Genomics Institute
Michio Hirano, MD, Columbia University Medical Center
Anne Lombès, MD, PhD, INSERM, Paris, France
Shamima Rahman, FRCP, FRCPCH, PhD, UCL Great Ormond Street Institute of Child Health, London, UK
Fernando Scaglia, MD, Baylor College of Medicine, Houston, TX, USA
Curt Scharfe, MD, PhD, Stanford University School of Medicine, Stanford, CA, USA
David Thorburn, PhD, FHGSA, FFSC (RCPA), Murdoch Children’s Research Institute, Victoria, Australia
Stephan Züchner, MD, PhD, Dr. John T. Macdonald Foundation, Miami, FL, USA
Imaging WG
David M. Mirsky, MD (Chair), Children’s Hospital Colorado, Aurora, CO, USA
Abigail Collins, MD, Children’s Hospital Colorado, Aurora, CO, USA
Andrea L. Gropman, MD, Children’s National Medical Center, Washington, DC, USA
Edward Yang, MD, Boston Children’s Hospital, Boston, MA, USA
Neurological Assessments WG
Bruce Cohen, MD, FAAN (Co-Chair), Akron Children’s Hospital, Akron, OH, USA
Ingrid Tein, MD, FRCP (Co-Chair), University of Toronto, ON, Canada
Laurence Bindoff, MD, PhD, University of Bergen and Haukeland
Michio Hirano, MD, Columbia University Medical Center
Matthew Klein, MD, MS, FACS, Edison Pharmaceuticals
Thomas Klopstock, MD, Friedrich-Baur-Institute, Munich, Germany
Saskia Koene, MD, Radboud University Nijmegen Medical Centre, Netherlands
Pascal Laforêt, MD, PhD, Groupe Hospitalier Pitié-Salpêtrière Assistance Publique-Hôpitaux de Paris, Paris, France
Margherita Milone, MD, PhD, Mayo School of Graduate Medical Education, Rochester, MN, USA
Jan Smeitink, MD, PhD, Radboud University Nijmegen Medical Centre, Netherlands
Patient-Reported Outcomes QoL WG
Mary Kay Koenig, MD (Co-Chair), University of Texas Health Science Center, Houston, TX, USA
Sumit Parikh, MD (Co-Chair), Cleveland Clinic, Cleveland, OH, USA
Cristy Balcells, RN MSN, Mito Action, Boston, MA, USA
Michio Hirano, MD, Columbia University Medical Center
Amel Karaa, MD, Massachusetts General Hospital, Boston, MA, USA
Robert McFarland, DoH, HEFCE, Newcastle University, UK
Russel Saneto, DO, Seattle Children’s Hospital, Seattle, WA, USA
Peter W. Stacpoole, MD, PhD, University of Florida, Gainesville, FL, USA
Philip Yeske, PhD, United Mitochondrial Disease Foundation
Vision WG
Patrick Yu-Wai-Man, MD, PhD, FRCPath, FRCOphth (Chair), Newcastle University and Moorfields Eye Hospital, UK
Piero Barboni, MD, University of Bologna, Bologna, Italy
Valerio Carelli, MD, PhD, University of Bologna School of Medicine, Bologna, Italy
Patrick Chinnery, MD, PhD, FRCPath, FRCP, University of Cambridge, UK
Graham E. Holder, PhD, Moorfields Eye Hospital, London, UK
John L. Keltner, MD, UC Davis School of Medicine, Sacramento, CA, USA
Tony Moore, MA, FRCS, FCROphth, FMedSci, Ophthalmology UCSF School of Medicine, San Francisco, CA
Alfredo A. Sadun, MD, PhD, Doheny Eye Institute/UCLA, Los Angeles, CA, USA
Claire Sheldon, MD, PhD, University of British Columbia, Vancouver, Canada
Marcela Votruba, MD, PhD, FRCOphth, Cardiff University, UK
NINDS CDE Team
Joanne Odenkirchen, MPH, NINDS CDE Project Officer, National Institutes of Health/National Institute of Neurological Disorders and Stroke, (NIH/NINDS), Bethesda, MD, USA
Vernon Anderson, PhD
Kathryn Camp MS, RD, Consultant to the NIH/Office of Dietary Supplements (ODS), Bethesda, MD, USA
William C. Copeland, PhD, National Institute of Environmental Health Sciences (NIEHS), Research Triangle Park, NC, USA
Kerry Goetz, MS, National Eye Institute (NEI)/NIH, Bethesda, MD, USA
Katrina A Gwinn, MD, NINDS-NIH, Bethesda, MD, USA
Petra Kaufmann, MD, MSc, National Center for Advancing Translational Sciences (NCATS)/ NIH, Bethesda, MD, USA
Danuta Krotoski, PhD, Eunice Kennedy Shriver NICHD/NIH, Bethesda, MD, USA
Lynne A. Wolfe, MS, PNP, BC, Undiagnosed Disease Program, NHGRI/NIH, Bethesda, MD, USA
Steven Zullo, PhD, NIH/NIBIB, Bethesda, MD, USA
Sherita Ala’i-Hansen, MS, The Emmes Corporation, Rockville, MD, USA
Muniza K. Sheikh, MS, MBA, The Emmes Corporation, Rockville, MD, USA
An erratum to this article is available at https://doi.org/10.1007/s10545-017-0081-z.
Rights and permissions
About this article
Cite this article
Karaa, A., Rahman, S., Lombès, A. et al. Common data elements for clinical research in mitochondrial disease: a National Institute for Neurological Disorders and Stroke project. J Inherit Metab Dis 40, 403–414 (2017). https://doi.org/10.1007/s10545-017-0035-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0035-5