Abstract
Objective
We aim to delineate the progression of cerebellar atrophy (the primary neuroimaging finding) in children with phosphomannomutase-deficiency (PMM2-CDG) by analyzing longitudinal MRI studies and performing cerebellar volumetric analysis and a 2D cerebellar measurement.
Methods
Statistical analysis was used to compare MRI measurements [midsagittal vermis relative diameter (MVRD) and volume] of children with PMM2-CDG and sex- and age-matched controls, and to determine the rate of progression of cerebellar atrophy at different ages.
Results
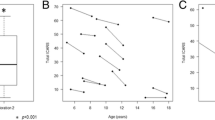
Fifty MRI studies of 33 PMM2-CDG patients were used for 2D evaluation, and 19 MRI studies were available for volumetric analysis. Results from a linear regression model showed that patients have a significantly lower MVRD and cerebellar volume compared to controls (p < 0.001 and p < 0.001 respectively). There was a significant negative correlation between age and MVRD for patients (p = 0.014). The rate of cerebellar atrophy measured by the loss of MVRD and cerebellar volume per year was higher at early ages (r = −0.578, p = 0.012 and r = −0.323, p = 0.48 respectively), particularly in patients under 11 years (p = 0.004). There was a significant positive correlation between MVRD and cerebellar volume in PMM2-CDG patients (r = 0.669, p = 0.001).
Conclusions
Our study quantifies a progression of cerebellar atrophy in PMM2-CDG patients, particularly during the first decade of life, and suggests a simple and reliable measure, the MVRD, to monitor cerebellar atrophy. Quantitative measurement of MVRD and cerebellar volume are essential for correlation with phenotype and outcome, natural follow-up, and monitoring in view of potential therapies in children with PMM2-CDG.


Similar content being viewed by others
Change history
09 June 2017
An erratum to this article has been published.
References
Antoun H, Villeneuve N, Gelot A, Panisset S, Adamsbaum C (1999) Cerebellar atrophy: an important feature of carbohydrate deficient glycoprotein syndrome type 1. Pediatr Radiol 29:194–198
Aronica E, van Kempen AA, van der Heide M et al (2005) Congenital disorder of glycosylation type Ia: a clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol 109:433–442
Barone R, Carrozzi M, Parini R et al (2015) A nationwide survey of PMM2-CDG in Italy: high frequency of a mild neurological variant associated with the L32R mutation. J Neurol 262:154–164
Eichler L, Bellenberg B, Hahn HK, Köster O, Schöls L, Lukas C (2011) Quantitative assessment of brain stem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status. AJNR Am J Neuroradiol 32:890–897
Feraco P, Mirabelli-Badenier M, Severino M et al (2012) The shrunken, bright cerebellum: a characteristic MRI finding in congenital disorders of glycosylation type 1a. AJNR Am J Neuroradiol 33:2062–2067
Freeze HH, Eklund EA, Ng BG, Patterson MC (2012) Neurology of inherited glycosylation disorders. Lancet Neurol 11:453–466
Freeze HH, Chong JX, Bamshad MJ, Ng BG (2014) Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet 94:161–175
Klockgether T, Skalej M, Wedekind D et al (1998) Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain 121:1687–1693
Lukas C, Bellenberg B, Köster O, Greschner S, Hahn HK (2011) A new sulcus-corrected approach for assessing cerebellar volume in spinocerebellar ataxia. Psychiatry Res 193:123–130
Pérez-Dueñas B, García-Cazorla A, Pineda M et al (2009) Long-term evolution of eight Spanish patients with CDG type Ia: typical and atypical manifestations. Eur J Paediatr Neurol 13:444–451
Serrano M, de Diego V, Muchart J et al (2015) Phosphomannomutase deficiency (PMM2-CDG): ataxia and cerebellar assessment. Orphanet J Rare Dis 10:138
Acknowledgments
We thank the patients and their families for their kind collaboration in all our projects, and particularly in this study.
We are grateful to the doctors and institutions from the Spanish national network for the study of glycosylation disorders for their collaboration. This work was supported by national grant PI14/00021 from the National Plan on I+D+I, cofinanced by ISC-III (Subdirección General de Evaluación y Fomento de la Investigación Sanitaria) and FEDER (Fondo Europeo de Desarrollo Regional) and IPT-2012- 0561-010000 from MINECO.
Collaborators of the CDG Spanish-Consortium
Sergio Aguilera-Albesa, MD PhD,1 Ramón Cancho Candela, MD PhD,2 Mª Llanos Carrasco Marina, MD,3 Francisco Carratalá, MD PhD,4 Mª Luz Couce, MD PhD,5 Ana Felipe, MD,6 Óscar García, MD,7 Mª Teresa García-Silva, MD PhD,8 Luis G Gutiérrez-Solana, MD PhD,9 Alfons Macaya, MD PhD,6 Mª Concepción Miranda, MD PhD,10 Laura López, MD,9 Eduardo López-Laso, MD PhD,11 M Pilar Póo, MD PhD,12 Pilar Quijada-Fraile, MD PhD8 Bernabé Robles, MD,PhD,13 Concepción Sierra-Córcoles, MD,14 Ramón Velázquez-Fragua, MD,15
1. Pediatric Neurology Unit, Department of Pediatrics, Navarra Hospital, Pamplona, Spain
2. Pediatric Neurology Unit. Pediatrics Department. Hospital Universitario Rio Hortega. Valladolid, Spain.
3. Neuropediatric Department, Pediatric Service, Hospital Universitario Severo Ochoa. Leganés, Madrid, Spain.
4. Pediatric Neurology Department, University Hospital Sant Joan d’Alacant, Spain.
5. Unit of Diagnosis and Treatment of Congenital Metabolic Diseases. Department of Pediatrics. Hospital Clínico Universitario de Santiago. CIBERER. Health Research Institute of Santiago de Compostela (IDIS), Santiago de Compostela, Spain.
6. Grup de Recerca en Neurologia Pediàtrica, Institut de Recerca Vall d’Hebron, Universitat Autònoma de Barcelona, Secció de Neurologia Pediàtrica, Hospital Universitari Vall d’Hebron, Barcelona, Spain.
7. Pediatric Department, Hospital Virgen de la Salud, Toledo, Spain.
8. Unidad de Enfermedades Mitocondriales-Enfermedades Metabólicas Hereditarias. Dpto. de Pediatría, Instituto de Investigación i+12, Hospital Universitario 12 de Octubre- CIBERER-ISCIII, Universidad Complutense de Madrid,Spain.
9. Unit of Child Neurology, Department of Pediatrics, Hospital Infantil Universitario Niño Jesús de Madrid, Madrid, Spain
10. Pediatric Neurology Unit, H.G.U Gregorio Marañón, Madrid, Spain
11. Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Pediatric Neurology Unit, Reina Sofia University Hospital, CIBERER, Córdoba, Spain.
12. Neuropediatric, Radiology and Clinical Biochemistry Departments, Hospital Sant Joan de Déu, Barcelona, Spain. U-703 Centre for Biomedical Research on Rare Diseases (CIBER-ER), Instituto de Salud Carlos III, Barcelona, Spain.
13. Neurology Department, Hospital General de Sant Boi, Parc Sanitari Sant Joan de Déu, Sant Boi, Barcelona, Spain.
14. Unidad de Neuropediatría, Hospital de Jaén, Jaén, Spain.
15. Pediatric Neurology Department, Hospital Universitario La Paz, Madrid, Spain.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Details of funding
The authors report no disclosure relevant to the manuscript. We did not have any sponsor in any phase of the study.
Conflict of interest
None.
Additional information
Communicated by: Jaak Jaeken
The original version of this article was revised: Due to an unfortunate error during the typesetting process, the collaborators were presented incorrectly.
An erratum to this article is available at https://doi.org/10.1007/s10545-017-0056-0.
Rights and permissions
About this article
Cite this article
de Diego, V., Martínez-Monseny, A.F., Muchart, J. et al. Longitudinal volumetric and 2D assessment of cerebellar atrophy in a large cohort of children with phosphomannomutase deficiency (PMM2-CDG). J Inherit Metab Dis 40, 709–713 (2017). https://doi.org/10.1007/s10545-017-0028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0028-4