Abstract
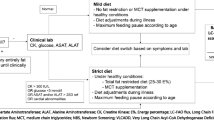
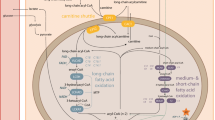
Children with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD) have a defect in the degradation of long-chain fatty acids and are at risk of hypoketotic hypoglycemia and insufficient energy production as well as accumulation of toxic fatty acid intermediates. Knowledge on substrate metabolism in children with LCHAD deficiency during fasting is limited. Treatment guidelines differ between centers, both as far as length of fasting periods and need for night feeds are concerned. To increase the understanding of fasting intolerance and improve treatment recommendations, children with LCHAD deficiency were investigated with stable isotope technique, microdialysis, and indirect calometry, in order to assess lipolysis and glucose production during 6 h of fasting. We found an early and increased lipolysis and accumulation of long chain acylcarnitines after 4 h of fasting, albeit no patients developed hypoglycemia. The rate of glycerol production, reflecting lipolysis, averaged 7.7 ± 1.6 µmol/kg/min, which is higher compared to that of peers. The rate of glucose production was normal for age; 19.6 ± 3.4 µmol/kg/min (3.5 ± 0.6 mg/kg/min). Resting energy expenditure was also normal, even though the respiratory quotient was increased indicating mainly glucose oxidation. The results show that lipolysis and accumulation of long chain acylcarnitines occurs before hypoglycemia in fasting children with LCHAD, which may indicate more limited fasting tolerance than previously suggested.


Similar content being viewed by others
Abbreviations
- AC:
-
Acylcarnitine
- BPM:
-
Beats Per Minute
- CV:
-
Coefficient of variation
- DHA:
-
Docosahexaenoic acid
- FAOD:
-
Fatty acid oxidation disorders
- GC-MS:
-
Gas chromatography–mass spectrometry
- GH:
-
Growth hormone
- LCHAD deficiency:
-
Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency
- LCT:
-
Long-chain triglyceride
- MCT fat:
-
Medium-chain triglyceride fat
- NEFA:
-
Non-esterified fatty acids
- REE:
-
Resting energy expenditure
- TAG:
-
Tri-acyl-glycerol
References
Arner P (2005) Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19:471–482
Arner P, Bolinder J (1991) Microdialysis of adipose tissue. J Intern Med 230:381–386
Arslanian SA, Kalhan SC (1994) Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 43(7):908–914
Bakermans AJ, Geraedts TR, Van Weeghel M et al (2011) Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging 4:558–565
Bier DM, Leake RD, Haymond MW et al (1977) Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 26:1016–1023
Bjorntorp P, Ostman J (1971) Human adipose tissue dynamics and regulation. Adv Metab Disord 5:277–327
Chaussain JL, Georges P, Olive G, Job JC (1974) Glycemic response to 24-hour fast in normal children and children with ketotic hypoglycemia: II. Hormonal and metabolic changes. J Pediatr 85:776–781
Chaussain JL, Georges P, Calzada L, Job JC (1977) Glycemic response to 24-hour fast in normal children: III. Influ age J Pediatr 91:711–714
Costin G, Kaufman FR, Brasel JA (1989) Growth hormone secretory dynamics in subjects with normal stature. J Pediatr 115(4):537–44
Danadian K, Lewy V, Janosky JJ, Arslanian S (2001) Lipolysis in African-American children: is it a metabolic risk factor predisposing to obesity? J Clin Endocrinol Metab 86:3022–3026
Den Boer ME, Wanders RJ, Morris AA, Ijlst L, Heymans HS, Wijburg FA (2002) Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics 109:99–104
Fahnehjelm KT, Holmstrom G, Ying L et al (2008) Ocular characteristics in 10 children with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: a cross-sectional study with long-term follow-up. Acta Ophthalmol 86:329–337
Fletcher AL, Pennesi ME, Harding CO, Weleber RG, Gillingham MB (2012) Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol Genet Metab 106:18–24
Food and Drug administration (1987) Guideline on Validation of the Limulus Amebocyte Lysate Test as End-Product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices Washington, DC: Food and Drug administration
Gillingham MB, Van Calcar S, Ney D, Wolff J, Harding CO (1999) Dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD). A case report and survey. J Inherit Metab Dis 22:123–131
Gillingham MB, Weleber RG, Neuringer M et al (2005) Effect of optimal dietary therapy upon visual function in children with long-chain 3-hydroxyacyl CoA dehydrogenase and trifunctional protein deficiency. Mol Genet Metab 86:124–133
Gillingham MB, Purnell JQ, Jordan J, Stadler D, Haqq AM, Harding CO (2007) Effects of higher dietary protein intake on energy balance and metabolic control in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab 90:64–69
Gillingham MB, Harding CO, Schoeller DA, Matern D, Purnell JQ (2013) Altered body composition and energy expenditure but normal glucose tolerance among humans with a long-chain fatty acid oxidation disorder. Am J Physiol Endocrinol Metab 305:E1299–E1308
Hagenfeldt L, Von Dobeln U, Holme E et al (1990) 3-Hydroxydicarboxylic aciduria-a fatty acid oxidation defect with severe prognosis. J Pediatr 116:387–392
Haglind CB, Halldin MU, Ask S et al (2013) Growth in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. JIMD Rep 8:81–90
Halldin MU, Brismar K, Tuvemo T, Gustafsson J (2002) Insulin sensitivity and lipolysis in adolescent girls with poorly controlled type 1 diabetes: effect of anticholinergic treatment. Clin Endocrincol (Oxf) 57(6):735–743
Halldin MU, Forslund A, Von Dobeln U, Eklund C, Gustafsson J (2007) Increased lipolysis in LCHAD deficiency. J Inherit Metab Dis 30:39–46
Haymond MW, Karl IE, Pagliara AS (1974) Ketotic hypoglycemia: an amino acid substrate limited disorder. J Clin Endocrinol Metab 38(4):521–530
Hellmer J, Arner P, Lundin A (1989) Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem 177(1):132–137
Hoffmann L, Seibt A, Herebian D, Spiekerkoetter U (2014) Monounsaturated 14:1n-9 and 16:1n-9 fatty acids but not 18:1n-9 induce apoptosis and necrosis in murine HL-1 cardiomyocytes. Lipids 49(1):25–37
Houten SM, Herrema H, Te Brinke H et al (2013) Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum Mol Genet 22(25):5249–5261
Kamel A, Norgren S, Persson B, Marcus C (1999) Insulin induced hypoglycaemia: comparison of glucose and glycerol concentrations in plasma and microdialysate from subcutaneous adipose tissue. Arch Dis Child 80:42–45
Kostyak JC, Kris-Etherton P, Bagshaw D, Delany JP, Farrell PA (2007) Relative fat oxidation is higher in children than adults. Nutr J 6:19
Levy-Marchal C, Arslanian S, Cutfield W et al (2010) Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 95(12):5189–5198
Lund AM, Skovby F, Vestergaard H, Christensen M, Christensen E (2010) Clinical and biochemical monitoring of patients with fatty acid oxidation disorders. J Inherit Metab Dis 33:495–500
Olpin SE, Clark S, Andresen BS et al (2005) Biochemical, clinical and molecular findings in LCHAD and general mitochondrial trifunctional protein deficiency. J Inherit Metab Dis 28:533–544
Persson B (1970) Determination of plasma acetoacetate and D-beta-hydroxybutyrate in new-born infants by an enzymatic fluorometric micro-method. Scand J Clin Lab Invest 25(1):9–18
Plock N, Kloft C (2005) Microdialysis—theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci 25:1–24
Potter BK, Little J, Chakraborty P et al (2012) Variability in the clinical management of fatty acid oxidation disorders: results of a survey of Canadian metabolic physicians. J Inherit Metab Dis 35(1):115–123
Raben MS, Hollenberg CH (1959) Effect of growth hormone on plasma fatty acids. J Clin Invest 38(3):484–488
Roe CR, Sweetman L, Roe DS, David F, Brunengraber H (2002) Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest 110:259–269
Saudubray JM, Martin D, De Lonlay P et al (1999) Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis 22:488–502
Schofield WN (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39(Suppl 1):5–41
Spiekerkoetter U, Lindner M, Santer R et al (2009) Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis 32(4):498–505
Spiekerkoetter U, Bastin J, Gillingham MB, Morris A, Wijburg F, Wilcken B (2010) Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis 33:555–561
Stumvoll M, Jacob S (1999) Multiple sites of insulin resistance: muscle, liver and adipose tissue. Exp Clin Endocrinol Diabetes 107:107–110
Sunehag AL, Ewald U, Gustafsson J (1996) Extremely preterm infants (<28 weeks) are capable of gluconeogenesis from glycerol on their first day of life. Pediatr Res 40(4):553–557
Sunehag AL, Treuth MS, Butte NF, Bier DM, Haymond MW (1998) Gluconeogenesis In Children Is Greater Than in Adolescents And Highly Reproducible. Pediatr Res 44:450–450
Sunehag AL, Treuth MS, Toffolo G et al (2001) Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: an evaluation of their reproducibility. Pediatr Res 50:115–123
Tucci S, Pearson S, Herebian D, Spiekerkoetter U (2013) Long-term dietary effects on substrate selection and muscle fiber type in very-long-chain acyl-CoA dehydrogenase deficient (VLCAD(−/−)) mice. Biochim Biophys Acta 1832:509–516
Tyni T, Majander A, Kalimo H, Rapola J, Pihko H (1996) Pathology of skeletal muscle and impaired respiratory chain function in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency with the G1528C mutation. Neuromuscul Disord 6(5):327–337
Tyni T, Kivela T, Lappi M, Summanen P, Nikoskelainen E, Pihko H (1998) Ophthalmologic findings in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency caused by the G1528C mutation: a new type of hereditary metabolic chorioretinopathy. Ophthalmology 105:810–824
Yokoyama S, Hirakawa H, Soeda J, Ueno S, Mitomi T (1997) Twenty-four-hour profile of growth hormone in cyclic nocturnal total parenteral nutrition. Pediatrics 100(6):973–976
Acknowledgments
We express our gratitude to the children and their families for taking part in the study. We are indebted to all our collaborators; Siw Siljerud, Sindra Isetun, Martin Engvall, Sten Salomonsson, Roger Olsson and Elisabeth Söderberg for skillful laboratory and technical expertise, nurses Jenny Gårdman, Jaana Nejla, Kerstin Ekbom, Karin Johansson, and Gunilla Wallin for excellent assistance and patient care. We thank associate Professor Jan Alm and Professor Antal Nemeth for valuable contributions. The study was supported by grants from the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institute and Uppsala County and Uppsala University, The Sven Jerring Foundation, The Samariten Foundation, The Child Care Society, The Gillberg foundation and Vera Ekström Foundation.
Compliance with ethical guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committee of Uppsala University, Sweden, decision number 2006/005, 2009-09-30).
Conflict of interest
None.
Human or animal studies
Informed consent was obtained from all families.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Piero Rinaldo
Rights and permissions
About this article
Cite this article
Haglind, C.B., Nordenström, A., Ask, S. et al. Increased and early lipolysis in children with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency during fast. J Inherit Metab Dis 38, 315–322 (2015). https://doi.org/10.1007/s10545-014-9750-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9750-3