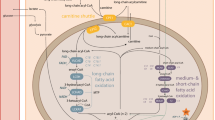
Summary
An increasing number of fatty acid oxidation defects are being detected owing to diagnostic improvements and a greater awareness among clinicians. The metabolic block leads to energy disruption, fatty infiltration, and toxic effects on organ functions exerted by β-oxidation metabolites. This investigation was undertaken to assess the influence of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency on lipolysis and energy turnover. We addressed the question whether the lipolysis and glucose production rates would be altered in the fasting state in a child with this disease. Lipolysis, glucose production and resting energy expenditure (REE) were studied in a 17-month-old girl with LCHAD deficiency and her healthy twin sister. Lipolysis and glucose production were determined after a 4–6 h fast by constant-rate infusion of [1,1,2,3,3-2H5]glycerol and [6,6-2H2]glucose and analysis by gas chromatography–mass spectrometry. REE was estimated by indirect calorimetry. The affected girl showed 50% higher lipolysis than did her sister, whereas the glucose production rates were similar. Plasma levels of dicarboxylic acids of 6–12 carbon atoms chain length, 3-hydroxy fatty acids of 6–18 carbon atoms chain length, total free fatty acids, and acylcarnitines were increased in the patient, as was REE. Since glucose production rates and plasma glucose levels were similar in the two girls, the increased lipolysis observed in the patient probably represents a compensatory mechanism for energy generation. This is achieved at the price of an augmented risk for fatty acid infiltration and toxic effects of β-oxidation intermediates. This highlights the importance of avoiding fasting in these patients.
Similar content being viewed by others
Abbreviations
- GH:
-
growth hormone
- LCHAD:
-
long-chain 3-hydroxyacyl-CoA dehydrogenase
- MCT fat:
-
medium-chain triacylglycerols
- NEFA:
-
nonesterified fatty acids
- REE:
-
resting energy expenditure
- SDS:
-
standard deviation score
- TAG:
-
triacylglycerols
References
Albertsson-Wikland K, Karlberg J (1994) Natural growth in children born small for gestational age with and without catch-up growth. Acta Padiatrica (Suppl) 399: 64–70.
Bang P, Eriksson U, Sara V, et al (1991) Comparison of acid ethanol extraction and acid gel filtration prior to IGF-I and IGF-II radioimmunoassays: improvement of determinations in acid ethanol extracts by the use of truncated IGF-I as a radioligand. Acta Endocrinologica (Copenh) 124: 620–629.
Benyon S (1998) Metabolic integration. In: Benyon S, Horton-Szar D, eds. Mosby’s Crash Course; Metabolism and Nutrition. London: Mosby International Ltd, 117–123.
Bier DM, Leake RD, Haymond MW et al (1977) Measurement of ‘true’ glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 26: 1016–1023.
Carlson MG, Snead WL, Campbell PJ (1994) Fuel and energy metabolism in fasting humans. Am J Clin Nutr 60: 29–36.
Cederblad G, Lindstedt S (1972) A method for the determination of carnitine in the picomole range. Clin Chim Acta 37: 235–243.
Clarke SD (2000) Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr 83: S59–S66.
Elia M, Zed C, Neale G, et al (1987) The energy cost of triglyceride–fatty acid recycling in nonobese subjects after an overnight fast and four days starvation. Metabolism 36: 251–255.
FAO/WHO/UNU (1985) Energy and protein requirements. WHO Technical report Series, 724. Geneva: WHO, 1–206.
Food and Drug Administration (1987) Guideline on Validation of Limulus Amebocyte Lysate Test as End Product Endotoxin Test for Human and Animal Parental Drugs, Biological Products, and Medical Devices. Washington, DC: Food and Drug Administration.
Hagenfeldt L, von Döbeln U, Holme E, et al (1990) 3-Hydroxydicarboxylic aciduria—a fatty acid oxidation defect with severe prognosis. J Pediatr 116: 387–392.
Halldin MU, Brismar K, Tuvemo T, et al (2002) Insulin sensitivity and lipolysis in adolescent girls with poorly controlled type 1 diabetes: effect of anticholinergic treatment. Clin Endocrinol 57: 735–743.
Kalhan SC, Bier DM, Savin SM, et al (1980) Estimation of glucose turnover and 13C recycling in the human newborn by simultaneous [I-13C]glucose and [6:6-2H2]glucose tracers. J Clin Endocrinol Metab 50: 456–460.
Lund AM, Dixon MA, Vreken P, et al (2003) What is the role of medium-chain triglycerides in the management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency? J Inherit Metab Dis 26: 353–360.
Niklasson A, Karlberg P (1993) Weight-for-height model in newborn Swedish infants. Acta Paediatr 87: 333–339.
Persson B (1970) Determination of plasma acetoacetate and D-β-hydroxybutyrate in new-born infants by an enzymatic fluorometric micro-method. Scand J Clin Lab Invest 25: 9–18.
Povoa G, Rooete A, Hall K (1984) Cross-reaction of serum somatomedin-binding protein in a radioimmunoassay developed for somatomedin binding protein isolated from human amniotic fluid. Acta Endocrinol (Copenh) 107: 563–570.
Raclot T (2003) Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog Lipid Res 42: 257–288.
Riudor E (1998) Neonatal onset in fatty acid oxidation disorders: how can we minimize morbidity and mortality? J Inherit Metab Dis 21:619–623.
Salway JG (1999a) Regulation of gluconeogenesis. In: Salway JG, ed. Metabolism at a Glance. Oxford: Blackwell Science, 72–73.
Salway JG (1999b) Regulation of fatty acid oxidation I: mobilization of fatty acids from storage in adipose tissue. In: Salway JG ed. Metabolism at a Glance. Oxford: Blackwell Science, 74–75.
Stumvoll M, Jacob S (1999) Multiple sites of insulin resistance: muscle, liver and adipose tissue. Exp Clin Endocrinol Diabetes 107: 107–110.
Sunehag A, Ewald U, Gustafsson J (1996) Extremely premature infants (<28,wk) are capable of gluconeogenesis from glycerol on their first day of life. Pediatr Res 40: 553–557.
Tyni T, Majander A, Kalimo H, et al (1996) Pathology of skeletal muscle and impaired respiratory chain function in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency with the G1528C mutation. Neuromusc Disord 5: 327–337.
Ventura FV, Ruiter JPN, Ijlst L, et al (1998) Lactic acidosis in long-chain fatty acid β-oxidation disorders. J Inherit Metab Dis 21: 645–654.
Wanders RJA, Ijlst L, van Gennip AH, et al (1990) Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis 13: 311–314.
Wanders RJA, Vreken P, den Boer MEJ, et al (1999) Disorders of mitochondrial fatty acyl-CoA β-oxidation. J Inherit Metab Dis 22: 442–487.
Weir JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Michael Bennett
Competing interests: None declared
References to electronic databases: Long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency: OMIM #609016; LCHAD: long-chain (S)-3-hydroxyacyl-CoA:NAD+ oxidoreductase, EC 1.1.1.211
Rights and permissions
About this article
Cite this article
Halldin, M.U., Forslund, A., von Döbeln, U. et al. Increased lipolysis in LCHAD deficiency. J Inherit Metab Dis 30, 39–46 (2007). https://doi.org/10.1007/s10545-006-0296-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-006-0296-x