Abstract
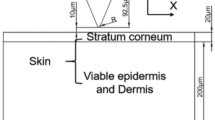
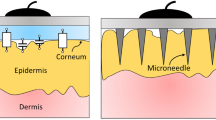
The conventional wet electrode for recording biosignals poses many inconveniences, as it requires an electrolytic gel that dries over time changing its electrical characteristics. The skin typically needs to be abraded when the electrode is applied to record high-quality signals, requiring assistance of trained personnel for the placement of the wet electrode. Alternative electrode designs to overcome these challenges often have difficulties recording small amplitude signals or their fabrication methods are complex and expensive. This research proposes a novel design and a simple fabrication method for a dry microneedle electrode for biosignal monitoring. The electrode can record electroencephalogram and electrocardiogram signals from a human subject without electrolytic gel and it does not require skin preparation such as abrasion, making it suitable for long term measurements as opposed to the wet electrode. When applied to the skin of a human subject with an impact inserter, the electrode has a lower impedance at the skin–electrode interface yielding better signal recording compared to application by hand. The selected electrode materials provides microneedles stiff enough to cross the outmost layer of the skin, while the flexible backing of the electrode has been designed to improve the conformation of the electrode to the rounded shape of the body. The proposed fabrication method for the electrode is based on a simple mold casting process that enables batch production while also reducing the time spent in a cleanroom and the use of expensive machinery.























Similar content being viewed by others
Data availability
Data available on request from the authors.
Code availability
Signal processing code available on request from the authors.
References
N.A. Alba, R.J. Sclabassi, M. Sun, X.T. Cui, IEEE Trans. Neural Syst. Rehabil. Eng. 18, 415 (2010)
M. Arai, Y. Kudo, N. Miki, in 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 3165–3168 (2015a)
M. Arai, Y. Nishinaka, N. Miki, Jpn. J. Appl. Phys. 54, 06FP14 (2015b)
J.Y. Baek, J.H. An, J.M. Choi, K.S. Park, S.H. Lee, Sens. Actuators, A 143, 423 (2008)
M. Böswald, K. Mende, W. Bernschneider, S. Bonakdar, H. Ruder, H. Kissler, E. Sieber, and J.-P. Guggenbichler, Infection 27, S38 (1999)
Y. M. Chi, S. R. Deiss, and G. Cauwenberghs, in 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks (IEEE, Berkeley, CA, 2009), pp. 246–250
S.P. Davis, B.J. Landis, Z.H. Adams, M.G. Allen, M.R. Prausnitz, J. Biomech. 37, 1155 (2004)
N.S. Dias, J.P. Carmo, A.F. da Silva, P.M. Mendes, J.H. Correia, Sens. Actuators, A 164, 28 (2010)
A. M. Dymond, T. L. Babb, L. E. Kaechele, and P. H. Crandall, Biomed. Sci. Instrum. 9, 1 (1972)
P. Griss, H.K. Tolvanen-Laakso, P. Merilainen, G. Stemme, IEEE Trans. Biomed. Eng. 49, 597 (2002)
K.M. Halprin, Br. J. Dermatol. 86, 14 (1972)
L.-S. Hsu, S.-W. Tung, C.-H. Kuo, Y.-J. Yang, Sensors 14, 12370 (2014)
H. Hua, W. Tang, X. Xu, D.D. Feng, L. Shu, Micromachines 10, 518 (2019)
W. F. Jackson, B. R. Duling, Circ. Res. 53, 105 (1983)
S. Kaushik, A.H. Hord, D.D. Denson, D.V. McAllister, S. Smitra, M.G. Allen, M.R. Prausnitz, Anesth. Analg. 92, 502 (2001)
R.S. Khandpur, in Compendium of Biomedical Instrumentation (John Wiley & Sons, Ltd, 2020), pp. 177–182
K. Kim, D.S. Park, H.M. Lu, W. Che, K. Kim, J.-B. Lee, C.H. Ahn, J. Micromech. Microeng. 14, 597 (2004)
N.K. Lamba, Polyurethanes in Biomedical Applications (Routledge, 2017)
L.A. Leslie, A. Geddes, L.E. Baker, Principles of Applied Biomedical Instrumentation (Wiley, 1975)
L.-D. Liao, I.-J. Wang, S.-F. Chen, J.-Y. Chang, C.-T. Lin, Sensors 11, 5819 (2011)
A. Lopez, P.C. Richardson, IEEE Trans. Biomed. Eng. BME-16, 99 (1969)
K. van der Maaden, E. Sekerdag, W. Jiskoot, J. Bouwstra, AAPS J 16, 681 (2014)
C. O’Mahony, F. Pini, A. Blake, C. Webster, J. O’Brien, and K. G. McCarthy, Sensors and Actuators A: Physical 186, 130 (2012)
O. Mecarelli, in Clinical Electroencephalography, edited by O. Mecarelli (Springer International Publishing, Cham, 2019), pp. 35–52
A.R. Mota, L. Duarte, D. Rodrigues, A.C. Martins, A.V. Machado, F. Vaz, P. Fiedler, J. Haueisen, J.M. Nóbrega, and C. Fonseca, Sens. Actuators A, Phys. 199, 310 (2013)
B. Mustin, B. Stoeber, Lab Chip 12, 4702 (2012)
W.C. Ng, H.L. Seet, K.S. Lee, N. Ning, W.X. Tai, M. Sutedja, J.Y.H. Fuh, X.P. Li, J. Mater. Process. Technol. 209, 4434 (2009)
Y. Nishinaka, R. Jun, G. S. Prihandana, N. Miki, Jpn. J. Appl. Phys. 52, 06GL10 (2013)
C. O’Mahony, Biomed Microdevices 16, 333 (2014)
J. Park, Y. Yoon, S. Choi, M.R. Prausnitz, M.G. Allen, IEEE Trans. Biomed. Eng. 54, 903 (2007)
J.-H. Park, M.G. Allen, M.R. Prausnitz, J. Control. Release 104, 51 (2005)
S.A. Ranamukhaarachchi, T. Schneider, S. Lehnert, L. Sprenger, J.R. Campbell, I. Mansoor, J.C.Y. Lai, K. Rai, J. Dutz, U.O. Häfeli, B. Stoeber, Macromol. Mater. Eng. 301, 306 (2016)
S.A. Ranamukhaarachchi, B. Stoeber, Biomed Microdevices 21, 100 (2019)
L. Ren, S. Xu, J. Gao, Z. Lin, Z. Chen, B. Liu, L. Liang, L. Jiang, Sensors (Basel) 18, (2018)
P. Shrestha, B. Stoeber, Physics of Fluids 32, 011905 (2020)
S. Silver, L.T. Phung, and G. Silver, J. Ind. Microbiol. Biotechnol. 33, 627 (2006)
S.R. Sinha, L.R. Sullivan, D. Sabau, D.S.J. Orta, K.E. Dombrowski, J.J. Halford, A.J. Hani, F.W. Drislane, M.M. Stecker, Neurodiagn. J. 56, 235 (2016)
E. Spinelli, M. Haberman, Physiol. Meas. 31, S183 (2010)
R. Splinter, editor, in Handbook of Physics in Medicine and Biology (CRC Press, 2010), pp. 259–268
T.J. Sullivan, S.R. Deiss, G. Cauwenberghs, in 2007 IEEE Biomedical Circuits and Systems Conference (2007), pp. 154–157
H. Tachikawa, N. Takano, K. Nishiyabu, N. Miki, Y. Ami, J. Solid Mech. Mater. Eng. 4, 470 (2010)
H. Tam, J.G. Webster, IEEE Trans. Biomed. Eng. BME-24, 134 (1977)
E. Touitou, B.W. Barry, Enhancement in Drug Delivery (2006)
A. Ueno, Y. Akabane, T. Kato, H. Hoshino, S. Kataoka, Y. Ishiyama, IEEE Trans. Biomed. Eng. 54, 759 (2007)
M.R. Ven Murthy, L. Carrier-Malhotra, in Protein-Dye Interactions: Developments and Applications, edited by M. A. Vijayalakshmi and O. Bertrand (Springer Netherlands, Dordrecht, 1989), pp. 316–330
F.J. Verbaan, S.M. Bal, D.J. van den Berg, J.A. Dijksman, M. van Hecke, H. Verpoorten, A. van den Berg, R. Luttge, J.A. Bouwstra, J. Control. Release 128, 80 (2008)
K. Vlach, J. Kijonka, F. Jurek, P. Vavra, P. Zonca, Sens. Actuators, B Chem. 245, 988 (2017)
R. Wang, X. Jiang, W. Wang, Z. Li, Sens. Actuators, B Chem. 244, 750 (2017)
R. Wang, W. Zhao, W. Wang, Z. Li, J. Microelectromech. Syst. 21, 1084 (2012)
N. Wilke, C. Hibert, J. O’Brien, A. Morrissey, Sens. Actuators, A 123–124, 319 (2005)
X. Xing, W. Pei, Y. Wang, X. Guo, H. Zhang, Y. Xie, Q. Gui, F. Wang, H. Chen, Sens. Actuators, A 270, 262 (2018)
W.C. Yang, Y.S. Huang, B.Y. Shew, C.C. Fu, J. Micromech. Microeng. 23, 035004 (2013)
Funding
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC).
We would like to acknowledge CMC Microsystems for the provision of products and services that facilitated this research, including CAD tools, test equipment and fabrication costs.
This research was undertaken, in part, with support from the Canada Research Chairs program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The research presented in this paper was carried out at the University of British Columbia, Canada. The Clinical Research Ethics Board (CREB) at The University of British Columbia classified all the tests that involve human subjects as quality improvement and did not require ethics board review or approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lozano, J., Stoeber, B. Fabrication and characterization of a microneedle array electrode with flexible backing for biosignal monitoring. Biomed Microdevices 23, 53 (2021). https://doi.org/10.1007/s10544-021-00583-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-021-00583-y