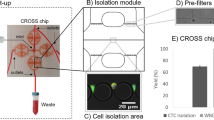
Abstract
Microfluidic, flow cytometry, and immunomagnetic methods for cancer cell isolation have heavily relied on the Epithelial Cellular Adhesion Molecule (EpCAM) for affinity separation. While EpCAM has been used extensively for circulating tumor cell isolation, it cannot be used to isolate non-epithelial cells. The human transferrin receptor (CD71) can also be used for cancer cell isolation and has the advantage that as an affinity target it can separate virtually any cancer cell type, regardless of disease origin. However, direct comparison of the capture ability of EpCAM and CD71 has not been reported previously. In this work, cell capture with both EpCAM and CD71 were studied using a novel higher-throughput herringbone cell separation microfluidic device. Five separation chip models were designed and the one with the highest capture efficiency (average 90 ± 10%) was chosen to compare antigen targets for cell capture. Multiple cancer cell lines including CCRF-CEM, PC-3 and MDA-MB-231 were tested for cell capture performance using both ligands (anti-CD71 and anti-EpCAM) in the optimized chip design. PC-3 and MDA-MB-231 cells were spiked into blood at concentrations ranging from 0.5%–10%. PC-3 cells were separated by anti-CD71 and anti-EpCAM with 32–37% and 31–50% capture purity respectively, while MDA-MB-231 were separated with 35–53% and 33–56% capture purity using anti-CD71 and anti-EpCAM for all concentrations. The enrichment factor for the lowest concentrations of cells in blood ranged from 66-74X. The resulting enrichment of cancer cells shows that anti-CD71 was found to be statistically similar to anti-EpCAM for epithelial cancer cells, while anti-CD71 can be further used for non-epithelial cells, where anti-EpCAM cannot be used.






Similar content being viewed by others
References
M. Asadian-Birjand, C. Biglione, J. Bergueiro, A. Cappelletti, C. Rahane, G. Chate, J. Khandare, B. Klemke, M.C. Strumia, M. Calderón, Transferrin decorated thermoresponsive nanogels as magnetic trap devices for circulating tumor cells. Macromol. Rapid Commun. 37(5), 439–445 (2016)
L. Bai, Y. Du, J. Peng, Y. Liu, Y. Wang, Y. Yang, C. Wang, Peptide-based isolation of circulating tumor cells by magnetic nanoparticles. J. Mater. Chem. B. 2(26), 4080–4088 (2014)
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018)
P. Chen, Y.-Y. Huang, K. Hoshino, X. Zhang, Multiscale immunomagnetic enrichment of circulating tumor cells: from tubes to microchips. Lab Chip 14(3), 446–458 (2014)
M. Cristofanilli, G.T. Budd, M.J. Ellis, A. Stopeck, J. Matera, M.C. Miller, J.M. Reuben, G.V. Doyle, W.J. Allard, L.W. Terstappen, Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351(8), 781–791 (2004)
H. Cui, B. Wang, W. Wang, Y. Hao, C. Liu, K. Song, S. Zhang, S. Wang, Frosted slides decorated with silica nanowires for detecting circulating tumor cells from prostate cancer patients. ACS Appl. Mater. Interfaces. 10(23), 19545–19553 (2018)
T.R. Daniels, T. Delgado, J.A. Rodriguez, G. Helguera, M.L. Penichet, The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 121(2), 144–158 (2006)
I. De Domenico, D.M. Ward, J. Kaplan, Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat. Rev. Mol. Cell Biol. 9(1), 72 (2008)
H. Drakesmith, E. Nemeth, T. Ganz, Ironing out ferroportin. Cell Metab. 22(5), 777–787 (2015)
M.T. Gabriel, L.R. Calleja, A. Chalopin, B. Ory, D. Heymann, Circulating tumor cells: a review of non–EpCAM-based approaches for cell enrichment and isolation. Clin. Chem. 62(4), 571–581 (2016)
Y. Gao, W. Li, Y. Zhang, D. Pappas, Cell Affinity Separations on Microfluidic Devices (Springer, In Affinity Chromatography, 2015), pp. 55–65
W.A. Jefferies, M.R. Brandon, S.V. Hunt, A.F. Williams, K.C. Gatter, D.Y. Mason, Transferrin receptor on endothelium of brain capillaries. Nature 312(5990), 162–163 (1984)
A. Jemal, F. Bray, M.M. Center, J. Ferlay, E. Ward, D. Forman, Global cancer statistics. CA Cancer J. Clin. 61(2), 69–90 (2011)
G. Khanal, S. Hiemstra, D. Pappas, Probing hypoxia-induced staurosporine resistance in prostate cancer cells with a microfluidic culture system. Analyst 139(13), 3274–3280 (2014)
S. Kim, S.-I. Han, M.-J. Park, C.-W. Jeon, Y.-D. Joo, I.-H. Choi, K.-H. Han, Circulating tumor cell microseparator based on lateral magnetophoresis and immunomagnetic nanobeads. Anal. Chem. 85(5), 2779–2786 (2013)
Y.J. Kim, Y.-T. Kang, Y.-H. Cho, Poly (ethylene glycol)-modified tapered-slit membrane filter for efficient release of captured viable circulating tumor cells. Anal. Chem. 88(16), 7938–7945 (2016)
R. Königsberg, E. Obermayr, G. Bises, G. Pfeiler, M. Gneist, F. Wrba, M. de Santis, R. Zeillinger, M. Hudec, C. Dittrich, Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 50(5), 700–710 (2011)
P. Li, Y. Gao, D. Pappas, Negative enrichment of target cells by microfluidic affinity chromatography. Anal. Chem. 83(20), 7863–7869 (2011)
W. Li, Y. Gao, D. Pappas, A complementary method to CD4 counting: measurement of CD4+/CD8+ T lymphocyte ratio in a tandem affinity microfluidic system. Biomed. Microdevices 17(6), 113 (2015)
W. Li, Y. Zhang, C.P. Reynolds, D. Pappas, Microfluidic separation of lymphoblasts for the isolation of acute lymphoblastic leukemia using the human transferrin receptor as a capture target. Anal. Chem. 89(14), 7340–7347 (2017)
Q. Liu, M. Wang, Y. Hu, H. Xing, X. Chen, Y. Zhang, Y. Chen, D. Bu, P. Zhu, The Usefulness of CD71 Expression by Flow Cytometry in the Diagnosis of Acute Leukemia. Am. Soc. Hematol. (2012)
V.J. Lyons, A. Helms, D. Pappas, The effect of protein expression on cancer cell capture using the Human Transferrin Receptor (CD71) as an affinity ligand. Anal. Chim. Acta 1076, 154–161 (2019)
V.J. Lyons, D. Pappas, Affinity separation and subsequent terminal differentiation of acute myeloid leukemia cells using the human transferrin receptor (CD71) as a capture target. Analyst 144(10), 3369–3380 (2019)
D.K. Marsee, G.S. Pinkus, H. Yu, CD71 (transferrin receptor) an effective marker for erythroid precursors in bone marrow biopsy specimens. Am. J. Clin. Pathol. 134(3), 429–435 (2010)
C. Mata, E. Longmire, D. McKenna, K. Glass, A. Hubel, Cell motion and recovery in a two-stream microfluidic device. Microfluid. Nanofluid. 8(4), 457–465 (2010)
D. Pappas, Microfluidics and cancer analysis: cell separation, cell/tissue culture, cell mechanics, and integrated analysis systems. Analyst 141(2), 525–535 (2016)
D. Pappas, Practical cell analysis. Wiley Online Library: (2010)
J.-M. Park, J.-Y. Lee, J.-G. Lee, H. Jeong, J.-M. Oh, Y.J. Kim, D. Park, M.S. Kim, H.J. Lee, J.H. Oh, Highly efficient assay of circulating tumor cells by selective sedimentation with a density gradient medium and microfiltration from whole blood. Anal. Chem. 84(17), 7400–7407 (2012)
S.A. Rahman, M. Yokoyama, S. Nishio, M. Takeuchi, Flow cytometric evaluation of transferrin receptor in transitional cell carcinoma. Urol. Res. 25(5), 325–329 (1997)
K. Rittenhouse-Diakun, H.V. Leengoed, J. Morgan, E. Hryhorenko, G. Paszkiewicz, J. Whitaker, A. Oseroff, The role of transferrin receptor (CD71) in photodynamic therapy of activated and malignant lymphocytes using the heme precursor δ-aminolevulinic acid (ALA). Photochem. Photobiol. 61(5), 523–528 (1995)
Y. Shen, X. Li, D. Dong, B. Zhang, Y. Xue, P. Shang, Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am. J. Cancer Res. 8(6), 916 (2018)
J.E. Shindelman, A.E. Ortmeyer, H.H. Sussman, Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int. J. Cancer 27(3), 329–334 (1981)
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2019. CA Cancer J. Clin. 69(1), 7–34 (2019)
A. Sin, S.K. Murthy, A. Revzin, R.G. Tompkins, M. Toner, Enrichment using antibody-coated microfluidic chambers in shear flow: Model mixtures of human lymphocytes. Biotechnol. Bioeng. 91(7), 816–826 (2005)
G. Spizzo, D. Fong, M. Wurm, C. Ensinger, P. Obrist, C. Hofer, G. Mazzoleni, G. Gastl, P. Went, EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J. Clin. Pathol. 64(5), 415–420 (2011)
K. Sterzyńska, B. Kempisty, P. Zawierucha, M. Zabel, Analysis of the specificity and selectivity of anti-EpCAM antibodies in breast cancer cell lines. Folia Histochem. Cytobiol. 50(4), 534–541 (2012)
S.L. Stott, C.H. Hsu, D.I. Tsukrov, M. Yu, D.T. Miyamoto, B.A. Waltman, S.M. Rothenberg, A.M. Shah, M.E. Smas, G.K. Korir, F.P. Jr. Floyd, A.J. Gilman, J.B. Lord, D. Winokur, S. Springer, D. Irimia, S. Nagrath, L.V. Sequist, R.J. Lee, K.J. Isselbacher, S. Maheswaran, D.A. Haber, M. Toner, Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U.S.A. 107(43), 18392–18397 (2010)
A.D. Stroock, S.K. Dertinger, A. Ajdari, I. Mezić, H.A. Stone, G.M. Whitesides, Chaotic mixer for microchannels. Science 295(5555), 647–651 (2002)
L. Tao, K. Zhang, Y. Sun, B. Jin, Z. Zhang, K. Yang, Anti-epithelial cell adhesion molecule monoclonal antibody conjugated fluorescent nanoparticle biosensor for sensitive detection of colon cancer cells. Biosens. Bioelectron. 35(1), 186–192 (2012)
B.T. van der Gun, L.J. Melchers, M.H. Ruiters, L.F. de Leij, P.M. McLaughlin, M.G. Rots, EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis 31(11), 1913–1921 (2010)
S. Wang, S. Sohrabi, J. Xu, J. Yang, Y. Liu, Geometry design of herringbone structures for cancer cell capture in a microfluidic device. Microfluid. Nanofluid. 20(11), 148 (2016)
P.T. Went, A. Lugli, S. Meier, M. Bundi, M. Mirlacher, G. Sauter, S. Dirnhofer, Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 35(1), 122–128 (2004)
B. Wickramaratne, M. Ivey, D. Pappas, Isolation of proliferating cells from whole blood using Human Transferrin Receptor in a two-stage separation system. Talanta 204, 731–738 (2019)
B. Wickramaratne, D. Pappas, Tandem microfluidic chip isolation of prostate and breast cancer cells from simulated liquid biopsies using CD71 as an affinity ligand. RSC Adv. 10(54), 32628–32637 (2020)
D. Woo, M. Yu, Circulating tumor cells as “liquid biopsies” to understand cancer metastasis. Transl. Res. 201, 128–135 (2018)
M. Yüce, N. Ullah, H. Budak, Trends in aptamer selection methods and applications. Analyst 140(16), 5379–5399 (2015)
Y. Zhang, W. Li, Y. Zhou, A. Johnson, A. Venable, A. Hassan, J. Griswold, D. Pappas, Detection of sepsis using CD64 expression in a microfluidic cell separation device. Analyst 143, 241–249 (2018)
Y. Zhang, V. Lyons, D. Pappas, Fundamentals of affinity cell separations. Electrophoresis 39(5–6), 732–741 (2018)
Y. Zhang, D. Pappas, Microfluidic cell surface antigen expression analysis using a single antibody type. Analyst 141(4), 1440–1447 (2016)
Y. Zhang, Y. Zhou, W. Li, V. Lyons, A. Johnson, A. Venable, J. Griswold, D. Pappas, Multiparameter affinity microchip for early sepsis diagnosis based on CD64 and CD69 expression and cell capture. Anal. Chem. 90(12), 7204–7211 (2018)
Y. Zhou, Z. Dong, H. Andarge, W. Li, D. Pappas, Nanoparticle modification of microfluidic cell separation for cancer cell detection and isolation. Analyst 145(1), 257–267 (2020)
Y. Zhou, Y. Zhang, A. Johnson, A. Venable, J. Griswold, D. Pappas, Combined CD25, CD64, and CD69 biomarker panel for flow cytometry diagnosis of sepsis. Talanta 191, 216–221 (2019)
Acknowledgments
This work was supported by a grant from the Cancer Research and Prevention Institute of Texas (CPRIT RP180862).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Zhou, Y., Wickramaratne, B. et al. A comparison of transferrin-receptor and epithelial cellular adhesion molecule targeting for microfluidic separation of cancer cells. Biomed Microdevices 23, 28 (2021). https://doi.org/10.1007/s10544-021-00566-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-021-00566-z