Abstract
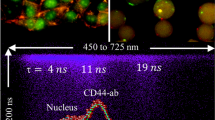
We demonstrate enhanced on-chip circulating tumor cell (CTC) detection through the incorporation of plasmonic-enhanced near-infrared (NIR) fluorescence screening. Specifically, the performance of plasmonic gold coated chips was evaluated on our previously reported immunomagnetic CTC capture system and compared to the performance of a regular chip. Three main performance metrics were evaluated: capture efficiency, capture reproducibility, and clinical efficacy. Use of the plasmonic chip to capture SK-BR-3 cells in PBS, resulted in a capture efficiency of 82%, compared to 76% with a regular chip. Both chips showed excellent capture reproducibility for all three cells lines evaluated (MCF-7, SK-BR-3, Colo 205) in both PBS and peripheral blood, with R2 values ranging from 0.983 to 0.996. Finally, performance of the plasmonic chip was evaluated on thirteen peripheral blood samples in patients with both breast and prostate cancer. The regular chip detected 2–8 cells per 5 mL of blood, while the plasmonic chip detected 8–85 cells per 5 mL of blood in parallel samples. In summary, we successfully demonstrate improved CTC capture and detection capabilities through use of plasmonic-enhanced near-infrared (NIR) fluorescence screening in both in vitro and ex vivo experiments. This work not only has the potential to improve clinical outcomes though improved CTC analysis, but also demonstrates successful interface design between plasmonic materials and cell capture for bioanalytical applications.





Similar content being viewed by others
References
W.J. Allard, J. Matera, M.C. Miller, M. Repollet, M.C. Connelly, C. Rao, A.G. Tibbe, J.W. Uhr, L.W. Terstappen, Clin. Cancer Res. 10, 2 (2004)
M.C. Chang, Y.T. Chang, J.Y. Chen, Y.M. Jeng, C.Y. Yang, Y.W. Tien, S.H. Yang, H.L. Chen, T.Y. Liang, C.F. Wang, E.Y. Lee, Y.C. Chang, W.H. Lee, Clin. Chem. 62, 3 (2016)
J.Y. Chen, W.S. Tsai, H.J. Shao, J.C. Wu, J.M. Lai, S.H. Lu, T.F. Hung, C.T. Yang, L.C. Wu, J.S. Chen, W.H. Lee, Y.C. Chang, PLoS One 11, 3 (2016)
M. Erkan, M. Kurtoglu, J. Kleeff, Expert Rev Gastroenterol Hepatol 10, 3 (2016)
D.L. Holliday, V. Speirs, Breast Cancer Res. 13, 4 (2011)
G. Hong, S.M. Tabakman, K. Welsher, Z. Chen, J.T. Robinson, H. Wang, B. Zhang, H. Dai, Angew. Chem. 50, 4644 (2011). https://doi.org/10.1002/anie.201100934
K. Hoshino, Y. Huang, N. Lane, M. Huebschman, J.W. Uhr, E.P. Frenkel, J.X. Zhang, Lab Chip 11, 20 (2011)
K. Hoshino, H. Chung, C. Wu, K. Rajendran, Y. Huang, P. Chen, K. Sokolov, J. Kim, X.J. Zhang, J. Circ. Biomark. 4, 11 (2015)
H.M. Kang, G.H. Kim, H.K. Jeon, D.H. Kim, T.Y. Jeon, D.Y. Park, H. Jeong, W.J. Chun, M.H. Kim, J. Park, M. Lim, T.H. Kim, Y.K. Cho, PLoS One 12, 6 (2017)
X. Li, T. Kuznetsova, N. Cauwenberghs, M. Wheeler, H. Maecker, J.C. Wu, F. Haddad, H. Dai, Proc. Natl. Acad. Sci. U. S. A. 114, 27 (2017)
T. Matsui, T. Onouchi, K. Shiogama, Y. Mizutani, K. Inada, F. Yu, D. Hayasaka, K. Morita, H. Ogawa, F. Mahara, Y. Tsutsumi, Acta Histochem. Cytochem. 48, 5 (2015)
P. Paterlini-Brechot, N.L. Benali, Cancer Lett. 253, 2 (2007)
V.H. Perez-Gonzalez, R.C. Gallo-Villanueva, S. Camacho-Leon, J.I. Gomez-Quiñones, J.M. Rodriguez-Delgado, S.O. Martinez-Chapa, IET Nanobiotechnol. 10, 5 (2016)
C. Printz, Cancer 123, 6 (2017)
M. Sahmani, M. Vatanmakanian, M. Goudarzi, N. Mobarra, M. Azad, Asian Pac. J. Cancer Prev. 17, 3 (2016)
T. Sawada, J. Araki, T. Yamashita, M. Masubuchi, T. Chiyoda, M. Yunokawa, K. Hoshi, S. Tao, S. Yamamura, S. Yatsushiro, K. Abe, M. Kataoka, T. Shimoyama, Y. Maeda, K. Kuroi, K. Tamura, T. Sawazumi, H. Minami, Y. Suda, F. Koizumi, EBioMedicine 11, 173 (2016). https://doi.org/10.1016/j.ebiom.2016.07.027
T.N. Seyfried, L.C. Huysentruyt, Crit. Rev. Oncog. 18, 1–2 (2013)
S.M. Tabakman, L. Lau, J.T. Robinson, J. Price, S.P. Sherlock, H. Wang, B. Zhang, Z. Chen, S. Tangsombatvisit, J.A. Jarrell, P.J. Utz, H. Dai, Nat. Commun. 2, 9 (2011)
A. Tadimety, A. Syed, Y. Nie, C.R. Long, K.M. Kreadya, J.X. Zhang, Integr. Biol. 9, 22 (2017)
A. Tadimety, A. Closson, C. Li, Y. Song, T. Shen, J.X. Zhang, Crit. Rev. Clin. Lab. Sci. 55, 3 (2018)
X. Wang, L. Shi, Q. Tao, H. Bao, J. Wu, D. Cai, F. Wang, Y. Zhao, G. Tian, Y. Li, C. Qao, H. Chen, J. Virol. Methods 167, 2 (2010)
C.H. Wu, Y.Y. Huang, K. Hoshino, P. Chen, H. Liu, E.P. Frenkel, J.X. Zhang, K.V. Sokolov, ACS Nano 7, 10 (2013)
J. Wu, X. Wei, J. Gan, J. Lou, T. Shen, B. Liu, J.X. Zhang, K. Qian, Adv. Funct. Mater. 26, 4016 (2016). https://doi.org/10.1002/adfm.201504184
B. Zhang, J.A. Jarrell, J.V. Price, S.M. Tabakman, Y. Li, M. Gong, G. Hong, J. Feng, P.J. Utz, H. Dai, PLoS One 8, 7 (2013)
Y. Zhang, X. Zhang, J. Zhang, B. Sun, L. Zheng, J. Li, S. Liu, G. Sui, Z. Yin, Cancer Biol Ther 17, 11 (2016)
R. Zhang, B. Le, W. Xu, K. Guo, X. Sun, H. Su, L. Huang, J. Huang, T. Shen, T. Liao, Y. Liang, J.X. Zhang, H. Dai, K. Qian. “Magnetic ‘squashing’ of circulating tumor cells on plasmonic substrates for ultrasensitive NIR fluorescence detection”. 2018
Y. Zhu, K. Kekalo, C. NDong, Y. Huang, F. Shubitidze, K.E. Krisword, I. Baker, J.X. Zhang, Adv. Funct. Mater. 26, 3953 (2016). https://doi.org/10.1002/adfm.201504176
Acknowledgements
The authors are grateful for the financial support from the National Institute of Health (NIH) Director’s Transformative Research Award (R01HL137157), NSF ECCS-1509369, and Norris Cotton Cancer Center Developmental Funds (Pilot Projects). We would also like to acknowledge Professor Hongjie Dai and his team from Stanford University for providing the expertise and resources on plasmonic detection.
Author information
Authors and Affiliations
Contributions
K.Q, T.S. and J.X.Z. conceived and designed the experiments; Y.S., YW.S., B.L. and R.Z. performed the experiment; WY.S.,LJ. W. and Y. X. collected healthy donors and patients; WY. S., A.B. and Y.S. wrote the paper; K.Q., T.S. and J X.Z. revised the paper.
Corresponding author
Ethics declarations
Conflicts of interest
NanoLite Systems has a technology licensing agreement with the corresponding author (John X. J. Zhang) for the CellRich™ system, but we have no other personal or financial conflicts of interests to disclose.
Rights and permissions
About this article
Cite this article
Shen, W., Song, Y., Burklund, A. et al. Combined immunomagnetic capture coupled with ultrasensitive plasmonic detection of circulating tumor cells in blood. Biomed Microdevices 20, 99 (2018). https://doi.org/10.1007/s10544-018-0333-1
Published:
DOI: https://doi.org/10.1007/s10544-018-0333-1