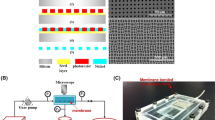
Abstract
Crossflow microfiltration of plasma from blood through microsieves in a microchannel is potentially useful in many biomedical applications, including clinically as a wearable water removal device under development by the authors. We report experiments that correlate filtration rates, transmembrane pressures (TMP) and shear rates during filtration through a microscopically high channel bounded by a low intrinsic resistance photolithographically-produced porous semiconductor membrane. These experiments allowed observation of erythrocyte behavior at the filtering surface and showed how their unique deformability properties dominated filtration resistance. At low filtration rates (corresponding to low TMP), they rolled along the filter surface, but at higher filtration rates (corresponding to higher TMP), they anchored themselves to the filter membrane, forming a self-assembled, incomplete monolayer. The incompleteness of the layer was an essential feature of the monolayer’s ability to support sustainable filtration. Maximum steady-state filtration flux was a function of wall shear rate, as predicted by conventional crossflow filtration theory, but, contrary to theories based on convective diffusion, showed weak dependence of filtration on erythrocyte concentration. Post-filtration scanning electron micrographs revealed significant capture and deformation of erythrocytes in all filter pores in the range 0.25 to 2 μm diameter. We report filtration rates through these filters and describe a largely unrecognized mechanism that allows stable filtration in the presence of substantial cell layers.








Similar content being viewed by others
References
M.H. Al-Malack, G. Anderson, Use of crossflow microfiltration in wastewater treatment. Water Res. 31(12), 3064–3072 (1997)
D. Barata, C. van Blitterswijk, P. Habibovic, High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater. 34, 1–20 (2016)
R.B. Bird, W.E. Stewart, and E.N. Lightfoot, Transport Phenomena. 2007: Wiley
W.F. Blatt, et al., Solute polarization and cake formation in membrane ultrafiltration: Causes, consequences, and control techniques, in Membrane Science and Technology. 1970, Springer. p. 47–97
T. Burnouf, Modern plasma fractionation. Transfus. Med. Rev. 21(2), 101–117 (2007)
P.B. Canham, The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J. Theor. Biol. 26(1), 61–81 (1970)
H. Carrère, F. Blaszkow, H.R. de Balmann, Modelling the clarification of lactic acid fermentation broths by cross-flow microfiltration. J. Membr. Sci. 186(2), 219–230 (2001)
C. Charcosset, Membrane processes in biotechnology: An overview. Biotechnol. Adv. 24(5), 482–492 (2006)
H. Chen et al., Combination of antibody-coated, physical-based microfluidic chip with wave-shaped arrays for isolating circulating tumor cells. Biomed. Microdevices 19(3), 66 (2017)
R.H. Davis, Modeling of fouling of crossflow microfiltration membranes. Sep. Purif. Methods 21(2), 75–126 (1992)
M. Dickson et al., A scalable, micropore, platelet rich plasma separation device. Biomed. Microdevices 14(6), 1095–1102 (2012)
D.A. Drew, J.A. Schonberg, G. Belfort, Lateral inertial migration of a small sphere in fast laminar flow through a membrane duct. Chem. Eng. Sci. 46(12), 3219–3224 (1991)
U. Frenander, A.S. Jönsson, Cell harvesting by cross-flow microfiltration using a shear-enhanced module. Biotechnol. Bioeng. 52(3), 397–403 (1996)
J. Garcia-Hartjes et al., Picomolar inhibition of cholera toxin by a pentavalent ganglioside GM1os-calix [5] arene. Org. Biomol. Chem. 11(26), 4340–4349 (2013)
P. Hodgson et al., Cake resistance and solute rejection in bacterial microfiltration: The role of the extracellular matrix. J. Membr. Sci. 79(1), 35–53 (1993)
I. Horcas et al., WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78(1), 013705 (2007)
H.W. Hou et al., Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 11(3), 557–564 (2009)
S.-W. Hu et al., Versatile microfluidic droplets array for bioanalysis. ACS Appl. Mater. Interfaces 7(1), 935–940 (2015)
K.F. Jensen, Microreaction engineering—Is small better? Chem. Eng. Sci. 56(2), 293–303 (2001)
H.M. Ji et al., Silicon-based microfilters for whole blood cell separation. Biomed. Microdevices 10(2), 251–257 (2008)
J. Kromkamp et al., A suspension flow model for hydrodynamics and concentration polarisation in crossflow microfiltration. J. Membr. Sci. 253(1–2), 67–79 (2005)
E.F. Leonard, C.S. Vassilieff, The deposition of rejected matter in membrane separation processes. Chem. Eng. Commun. 30(3–5), 209–217 (1984)
H. Li et al., Observation of deposition and removal behaviour of submicron bacteria on the membrane surface during crossflow microfiltration. J. Membr. Sci. 217(1), 29–41 (2003)
M.R. Mackley, N.E. Sherman, Cross-flow cake filtration mechanisms and kinetics. Chem. Eng. Sci. 47(12), 3067–3084 (1992)
J.L. Madrigal et al., Microgels produced using microfluidic on-chip polymer blending for controlled released of VEGF encoding lentivectors. Acta Biomater. 69, 265 (2018)
M.S. Maria et al., Development of a microfluidic device for cell concentration and blood cell-plasma separation. Biomed. Microdevices 17(6), 115 (2015)
A.B. Martin et al., Development of a high-throughput magnetic separation device for malaria-infected erythrocytes. Ann. Biomed. Eng. 45(12), 2888–2898 (2017)
M. Mercier et al., Yeast suspension filtration: Flux enhancement using an upward gas/liquid slug flow—Application to continuous alcoholic fermentation with cell recycle. Biotechnol. Bioeng. 58(1), 47–57 (1998)
F.M. Morel, R.F. Baker, H. Wayland, Quantitation of human red blood cell fixation by glutaraldehyde. J. Cell Biol. 48(1), 91–100 (1971)
X. Peng et al., Ultrafast permeation of water through protein-based membranes. Nat. Nanotechnol. 4(6), 353–357 (2009)
M.C. Porter, Concentration polarization with membrane ultrafiltration. Ind. Eng. Chem. Prod. Res. Dev. 11(3), 234–248 (1972)
J. Rho et al., Magnetic nanosensor for detection and profiling of erythrocyte-derived microvesicles. ACS Nano 7(12), 11227–11233 (2013)
S. Ripperger, J. Altmann, Crossflow microfiltration–state of the art. Sep. Purif. Technol. 26(1), 19–31 (2002)
R.O. Rodrigues et al., A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 17(6), 108 (2015)
C.A. Romero, R.H. Davis, Transient model of crossflow microfiltration. Chem. Eng. Sci. 45(1), 13–25 (1990)
N. Rossignol et al., Membrane technology for the continuous separation microalgae/culture medium: Compared performances of cross-flow microfiltration and ultrafiltration. Aquac. Eng. 20(3), 191–208 (1999)
G. Segre, A. Silberberg, Behaviour of macroscopic rigid spheres in Poiseuille flow part 1. Determination of local concentration by statistical analysis of particle passages through crossed light beams. J. Fluid Mech. 14(01), 115–135 (1962)
S. Sethi, M.R. Wiesner, Modeling of transient permeate flux in cross-flow membrane filtration incorporating multiple particle transport mechanisms. J. Membr. Sci. 136(1), 191–205 (1997)
J. Sibbitts et al., Cellular analysis using microfluidics. Anal. Chem. 90(1), 65–85 (2017)
R. Silva et al., Rapid prototyping and parametric optimization of plastic acoustofluidic devices for blood–bacteria separation. Biomed. Microdevices 19(3), 70 (2017)
E. Sollier et al., Fast and continuous plasma extraction from whole human blood based on expanding cell-free layer devices. Biomed. Microdevices 12(3), 485–497 (2010)
N.V. Tolan et al., Personalized metabolic assessment of erythrocytes using microfluidic delivery to an array of luminescent wells. Anal. Chem. 81(8), 3102–3108 (2009)
C.J. van Rijn, Nano and micro engineered. Membr. Technol. 10 (2004) Elsevier
C.J. van Rijn et al., Microsieves made with laser interference lithography for micro-filtration applications. J. Micromech. Microeng. 9(2), 170 (1999)
V. VanDelinder, A. Groisman, Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Anal. Chem. 78(11), 3765–3771 (2006)
V. VanDelinder, A. Groisman, Perfusion in microfluidic cross-flow: Separation of white blood cells from whole blood and exchange of medium in a continuous flow. Anal. Chem. 79(5), 2023–2030 (2007)
P. Vasseur, R. Cox, The lateral migration of a spherical particle in two-dimensional shear flows. J. Fluid Mech. 78(02), 385–413 (1976)
C.S. Vassilieff, Convective model of cross-flow microfiltration. Adv. Colloid Interf. Sci. 40(0), 1–36 (1992)
N.A. Wagdare et al., High throughput vegetable oil-in-water emulsification with a high porosity micro-engineered membrane. J. Membr. Sci. 347(1–2), 1–7 (2010)
W.-T. Wu et al., Design of microfluidic channels for magnetic separation of malaria-infected red blood cells. Microfluid. Nanofluid. 20(2), 41 (2016)
T. Yaginuma et al., Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 7(5), 054110 (2013)
H. Yamazoe et al., Fabrication of patterned cell co-cultures on albumin-based substrate: Applications for microfluidic devices. Acta Biomater. 6(2), 526–533 (2010)
D.C. Yeo et al., Interference-free micro/nanoparticle cell engineering by use of high-throughput microfluidic separation. ACS Appl. Mater. Interfaces 7(37), 20855–20864 (2015)
A. Zydney, C. Colton, Continuous flow membrane plasmapheresis: Theoretical models for flux and hemolysis prediction. ASAIO J. 28, 408 (1982) & hyhen
A.L. Zydney, C.K. Colton, A concentration polarization model for the filtrate flux in cross-flow microfiltration of particulate suspensions. Chem. Eng. Commun. 47(1–3), 1–21 (1986)
A.L. Zydney, W.M. Saltzman, C.K. Colton, Hydraulic resistance of red cell beds in an unstirred filtration cell. Chem. Eng. Sci. 44(1), 147–159 (1989)
Acknowledgements
Support for this work was provided in part by Grant 1R21HL088162 from the National Institute of Health, and Vizio Medical Devices, LLC. The authors also thank Columbia Medical Center Blood Bank and blood donors. We acknowledge gratefully the assistance of Dr. Robert von Gutfeld and to our whole medical team, especially Dr.Stanley Cortell and most especially the late Dr. James Jones.
Author information
Authors and Affiliations
Corresponding author
Additional information
James P. Jones died before publication of this work was completed.
Topical area: Microfluidics, Separations: Materials, Devices, and Processes, Artificial Organs.
Appendix
Appendix
1.1 Nomenclature
B Half height of the channel (m)
J Permeate flux (m/s)
L Channel Length (m)
W Channel width (m)
ΔP Pressure drop across the channel (torr)
QF Volumetric flowrate of the permeate (i.e. Filtration rate) (cm3/min)
Qm Volumetric flowrate in main channel (cm3/min)
TMP Transmembrane pressure (torr)
a Particle radius (m)
dp Particle diameter
Am Membrane area (m2)
n0 number of pores per membrane
n number of open pores per membrane
FL Lift force (N)
FVDW Van der Waals force (N)
h Distance between the particle center and the membrane surface (cm)
Jf Filtrate flux (cm3/cm2-min)
KL Dimensionless constant in lift force
P Local hydrostatic pressure (torr)
P1 Outlet pressure (torr)
P2 Inlet pressure (torr)
P3 Filtrate pressure (torr)
mp Mass of particles in the cake (kg)
RM Resistance of membrane
RL Resistance of erythrocyte monolayer
RC Resistance of cake layer
1.2 Greek symbols
α Specific resistance of cake deposit
ε Fractional voidage of cake deposit
ρ fluid density (kg/m3)
γw Nominal wall shear rate (1/s)
τw Wall shear stress
ρp Particle density
η Viscosity of the media
μw Viscosity of pure water
1.3 Supporting data
Transmembrane pressure profile: Initial rising slope represents the time to reach steady state (section A). Plateau represents stable filtration under subcritical TMP (section B). Steep rising represents unstable filtration with cellular accumulation and jamming of the filtering membrane when filtrate rate was increased above critical TMP (section C). TMP drops back to 0 Torr when filtrate pump is stopped (at 560 s)
Rights and permissions
About this article
Cite this article
Amar, L.I., Guisado, D., Faria, M. et al. Erythrocyte fouling on micro-engineered membranes. Biomed Microdevices 20, 55 (2018). https://doi.org/10.1007/s10544-018-0297-1
Published:
DOI: https://doi.org/10.1007/s10544-018-0297-1