Abstract
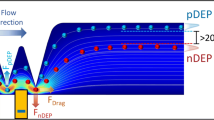
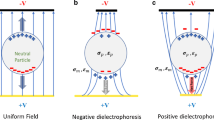
In this work, a novel force equilibrium method called distributed dielectrophoretic cytometry (2DEP cytometry) was developed. It uses a dielectrophoresis (DEP)-induced vertical translation of live cells in conjunction with particle image velocimetry (PIV) in order to measure probabilistic distribution of DEP forces acting on an entire cell population. The method is integrated in a microfluidic device. The bottom of the microfluidic channel is lined with an interdigitated electrode array. Cells passing through the micro-channel are acted on by sedimentation forces, while DEP forces either oppose sedimentation, support sedimentation, or neither, depending on the dielectric (DE) signatures of the cells. The heights at which cells stabilize correspond to their DE signature and are measured indirectly using PIV, which enables simultaneous and high-throughput collection of hundreds of single-cell responses in a single PIV frame. The system was validated using polystyrene micro-particles. Preliminary experimental data quantify the DE signatures of immortalized myelogenous leukemia cell lines K562 and KG1. We show DEP-induced cell translation along the parabolic velocity profile can be measured by PIV with sub-micron precision, enabling identification of individual cell DE signatures. DE signatures of the selected cell lines are distinguishable. Throughput of the method enables measurement of DE signatures at 10 different frequencies in almost real time.













Similar content being viewed by others
References
G. Bahrieh, M. Erdem, E. Özgür, U. Gündüz, H. Külah, RSC Adv. 4(85) (2014)
K. Braasch, M. Nikolic-Jaric, T. Cabel, E. Salimi, G.E. Bridges, D.J. Thomson, M. Butler, Biotechnol. Bioeng. 110(11) (2013)
S. Chin, M.P. Hughes, H.M. Coley, F.H. Labeed, Int. J. Nanomedicine 1(3) (2006)
H.M. Coley, F.H. Labeed, H. Thomas, M.P. Hughes, BBA-Gen. Subjects 1770(4) (2007)
L. Duncan, H. Shelmerdine, M.P. Hughes, H.M. Coley, Y. Hübner, F.H. Labeed, Phys. Med. Biol. 53(2) (2007)
P.R.C. Gascoyne, R. Pethig, J. Satayavivad, F.F. Becker, M. Ruchirawat, BBA-Biomembranes 1323(2) (1997)
P.R.C. Gascoyne, S. Shim, J. Noshari, F.F. Becker, K. Stemke-Hale, Electrophoresis 34(7) (2013)
T. Honegger, K. Berton, E. Picard, D. Peyrade, Appl. Phys. Lett. 98(18) (2011)
Y. Huang, X.B. Wang, F.F. Becker, P.R.C. Gascoyne, Biophys. J. 73 (1997)
T.B. Jones, IEEE Eng. Med. Biol. Mag. (2003)
F.H. Labeed, Biophys. J. 85 (2003)
A. Menachery, S. Chappell, R.J. Errington, D. Morris, P.J. Smith, M. Wiltshire, E. Furon, J. Burt., NSTI-Nanotech (UNE) 13 (2016)
H.S. Moon, K. Kwon, S.I. Kim, H. Han, J. Sohn, S. Lee, H.I. Jung, Lab Chip 11(6) (2011)
H.J. Mulhall, A. Cardnell, K.F. Hoettges, F.H. Labeed, M.P. Hughes, Integr. Biol. 7(11) (2015)
M. Nikolic-Jaric, S.F. Romanuik, G.A. Ferrier, T. Cabel, E. Salimi, D.B. Levin, G.E. Bridges, D.J. Thomson, Biomicrofluidics 6(2) (2012)
I.S. Noorjannah, Microelectron. Eng. 97 (2012)
I.S. Park, J. Lee, G. Lee, K. Nam, T. Lee, W.J. Chang, H. Kim, S.Y. Lee, J. Seo, D.S. Yoon, Anal. Chem. 87(12) (2015)
R. Pethig, Biomicrofluidics 4(2) (2010)
K. Salimi, K. Braasch, M. Butler, D.J. Thomson, G.E. Bridges, Biomicrofluidics 10(1) (2016)
S. Shim, P.R.C. Gascoyne, Cancers 6(1) (2014)
S. Shim, K. Stemke-Hale, A.M. Tsimberidou, J. Noshari, T.E. Anderson, P.R.C. Gascoyne, Biomicrofluidics 7(1) (2013a)
S. Shim, K. Stemke-Hale, J. Noshari, F.F. Becker, P.R.C. Gascoyne, Biomicrofluidics 7(1) (2013b)
A. Sigal, R. Milo, A. Cohen, N. Geva-Zatorsky, Y. Klein, Y. Liron, N. Rosenfeld, T. Danon, N. Perzov, U. Alon, Nature 444 (2006)
R. Soffe, S. Baratchi, S.Y. Tang, M. Nasabi, P. McIntyre, A. Mitchell, K. Khoshmanesh, Nature 5 (2015)
M.S. Talary, R. Pethig, Iet Nanobiotechnol. 1(1) (2007)
A. Tzur, R. Kafri, V.S. LeBleu, G. Lahav, M.W. Kirschner, Science 325(167) (2009)
M.D. Vahey, J. Voldman, Single-Cell Analysis: Methods and Protocols, (2012)
J. Vykoukal, D.M. Vykoukal, S. Freyberg, E.U. Alt, P.R.C. Gascoyne, Lab Chip 8(8) (2008)
X. Wang, F.F. Becker, P.R.C. Gascoyne, 1564(2) (2002)
J. Yang, Y. Huang, X.B. Wang, F.F. Becker, P.R.C. Gascoyne, Anal. Chem. 71(5) (1999)
J. Yang, Y. Huang, X.B. Wang, F.F. Becker, P.R.C. Gascoyne, Biophys. J. 78(5) (2000)
Acknowledgments
This work was supported by the Ministry of Education Youth and Sport of the Czech Republic within the PUNTIS project no. LO1506, Bone Marrow Transplant Foundation, project “Yeasts on chip” and the institutional support of the University of West Bohemia, MS POST DOC 2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fikar, P., Georgiev, V., Lissorgues, G. et al. 2DEP cytometry: distributed dielectrophoretic cytometry for live cell dielectric signature measurement on population level. Biomed Microdevices 20, 12 (2018). https://doi.org/10.1007/s10544-017-0253-5
Published:
DOI: https://doi.org/10.1007/s10544-017-0253-5