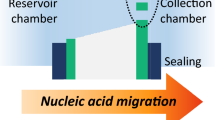
Abstract
We describe the development and evaluation of a rotary-based platform with multiple disposable fluidic modules for simultaneous automatic nucleic acid (NA) isolation from up to 24 biological samples. The procedure is performed inside insulated individual disposable modules, which minimizes both the risk of infection of personnel and laboratory cross-contamination. Each module is a segment of a circular cylinder containing a leak-proof inlet port for sample input, reservoirs with lyophilized chemicals and solvents, fluidic channels, stoppers, valves, a waste reservoir and an outlet port equipped with the standard micro test tube for NA collection. The entire platform, apart from the rotor that accommodates 24 modules, consists of functional elements that provide spinning of the rotor, reagent mixing, pressure delivery, and heating of reaction mixtures. The transfer of the reaction mixtures inside the modules is performed either with rotation of the rotor or with excessive air pressure applied to the module’s reservoirs. The entire process takes less than 40 min, starting from the sample loading to the recovery of the purified NA, and it allows NA isolation both from bacterial cells and viral particles. The feasibility and reproducibility of the developed platform was demonstrated by the NA isolation from suspensions of Bacillus thuringiensis and Mycobacterium tuberculosis cells within a concentration range of 108 to 102 cells/ml. Isolation of NAs from blood plasma samples with varying concentration of hepatitis B and C viruses from 107 to 102 particles/ml were also successful. The purity and integrity of the extracted NAs were both reliable for performing quantitative PCR.








Similar content being viewed by others
References
S. Berensmeier, Magnetic particles for the separation and purification of nucleic acids. Appl Microbiol Biotechnol 73(3), 495–504 (2006)
R. Boom, C.J. Sol et al., Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28(3), 495–503 (1990)
T. Brenner, T. Glatzel et al., Frequency-dependent transversal flow control in centrifugal microfluidics. Lab Chip 5(2), 146–150 (2005)
Y.K. Cho, J.G. Lee et al., One-step pathogen specific DNA extraction from whole blood on a centrifugal microfluidic device. Lab Chip 7(5), 565–573 (2007)
D. Eicher, C.A. Merten, Microfluidic devices for diagnostic applications. Expert Rev Mol Diagn 11(5), 505–519 (2011)
M.A. Gijs, F. Lacharme et al., Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev 110(3), 1518–1563 (2010)
R. Gorkin, J. Park et al., Centrifugal microfluidics for biomedical applications. Lab Chip 10(14), 1758–1773 (2010)
D. Gryadunov, V. Mikhailovich et al., Evaluation of hybridisation on oligonucleotide microarrays for analysis of drug-resistant Mycobacterium tuberculosis. Clin Microbiol Infect 11(7), 531–539 (2005)
D. Gryadunov, E. Dementieva et al., Gel-based microarrays in clinical diagnostics in Russia. Expert Rev Mol Diagn 11(8), 839–853 (2011)
S. Haeberle, Pausch, S. Burger, R. Lutz, S. von Stetten, F. Zengerle, R. Ducrée, J. Automation of nucleic acid extraction by a Coriolis-force actuated droplet router in μTAS. Eleventh International Conference on Miniaturized Systems for Chemistry and Life Sciences, Paris, France, (2007)
M.M. Hoehl, M. Weissert et al., Centrifugal LabTube platform for fully automated DNA purification and LAMP amplification based on an integrated, low-cost heating system. Biomed Microdevices 16(3), 375–385 (2014)
J.H. Jung, B.H. Park et al., A microbead-incorporated centrifugal sample pretreatment microdevice. Lab Chip 13(17), 3383–3388 (2013)
D.A. Khodakov, D.D. Mamaev et al., Microfluidic module for automated isolation and purification of nucleic acids from biological samples. Dokl Biochem Biophys 435, 291–294 (2010)
H. Kido, M. Micic et al., A novel, compact disk-like centrifugal microfluidics system for cell lysis and sample homogenization. Colloids Surf B Biointerfaces 58(1), 44–51 (2007)
S. Kitano, J. Myers et al., A novel fully automated molecular diagnostic system (AMDS) for colorectal cancer mutation detection. PLoS One 8(5), e62989 (2013)
A. Kloke, A.R. Fiebach et al., The LabTube - a novel microfluidic platform for assay automation in laboratory centrifuges. Lab Chip 14(9), 1527–1537 (2014)
M. Madou, J. Zoval et al., Lab on a CD. Annu Rev Biomed Eng 8, 601–628 (2006)
D.D. Mamaev, D.A. Khodakov et al., Method for automated extraction and purification of nucleic acids and its implementation in microfluidic system. Prikl Biokhim Mikrobiol 47(2), 231–240 (2011)
D. Mark, S. Haeberle et al., Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev 39(3), 1153–1182 (2010)
B.H. Park, J.H. Jung et al., A rotary microsystem for simple, rapid and automatic RNA purification. Lab Chip 12(20), 3875–3881 (2012)
K. Penuelas-Urquides, L. Villarreal-Trevino et al., Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz J Microbiol 44(1), 287–289 (2013)
S. Saleh-Lakha, J.T. Trevors, Perspective: microfluidic applications in microbiology. J Microbiol Methods 82(1), 108–111 (2010)
J. Siegrist, R. Gorkin et al., Validation of a centrifugal microfluidic sample lysis and homogenization platform for nucleic acid extraction with clinical samples. Lab Chip 10(3), 363–371 (2010)
Y. Song, Y.Y. Huang, X. Liu, X. Zhang, M. Ferrari, L. Qin, Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol 32(3), 132–139 (2014)
O. Strohmeier, A. Emperle et al., Centrifugal gas-phase transition magnetophoresis (GTM)–a generic method for automation of magnetic bead based assays on the centrifugal microfluidic platform and application to DNA purification. Lab Chip 13(1), 146–155 (2013)
E. van Zanten, G.J. Wisselink et al., Comparison of the QIAsymphony automated nucleic acid extraction and PCR setup platforms with NucliSens easyMAG and Corbett CAS-1200 liquid handling station for the detection of enteric pathogens in fecal samples. J Microbiol Methods 84(2), 335–340 (2011)
J. Verheyen, R. Kaiser et al., Extraction of viral nucleic acids: comparison of five automated nucleic acid extraction platforms. J Clin Virol 54(3), 255–259 (2012)
W. Weaver, H. Kittur, M.M. Dhar, D. Di Carlo, Research highlights: microfluidic point-of-care diagnostics. Lab Chip 14(12), 1962–1965 (2014)
J. Wen, L.A. Legendre et al., Purification of nucleic acids in microfluidic devices. Anal Chem 80(17), 6472–6479 (2008)
G. Yang, D.E. Erdman et al., Comparison of commercial systems for extraction of nucleic acids from DNA/RNA respiratory pathogens. J Virol Methods 171(1), 195–199 (2011)
Acknowledgments
This project has been funded partially by a Molecular and Cell Biology grant from the Russian Academy of Sciences and with subsidy #14.607.21.0065 from the Ministry of Science and Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mamaev, D., Shaskolskiy, B., Dementieva, E. et al. Rotary-based platform with disposable fluidic modules for automated isolation of nucleic acids. Biomed Microdevices 17, 18 (2015). https://doi.org/10.1007/s10544-014-9920-y
Published:
DOI: https://doi.org/10.1007/s10544-014-9920-y