Abstract
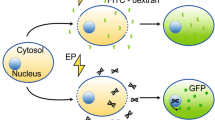
In this study, a new microelectrode assembly based on spiral geometry applicable to in situ electroporation of adherent cell monolayers on standard multiwell plates is presented. Furthermore, the structure is specially conceived to perform electrical impedance spectroscopy (EIS) measurements during electroporation. Its performance for cell membrane permeabilization is tested with a fluorescent probe. Gene electrotransfer is also assayed using a plasmid DNA encoding GFP in four different cell lines (CHO, HEK293, 3T3-L1 and FTO2B). Additionally, siRNA α-GFP electrotransfection is tested in GFP gene-expressing CHO cells. Our data show considerable differences between permeabilization and gene transfer results and cell line dependence on gene expression rates. Successful siRNA electro-mediated delivery is also achieved. We demonstrate the applicability of our device for electroporation-mediated gene transfer of adherent cells in standard laboratory conditions. Finally, electrical impedance measurements during electroporation of CHO and 3T3-L1 cells are also given.








Similar content being viewed by others
References
F. Andre, L.M. Mir, DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Ther. 11(Suppl 1), S33–S42 (2004)
A.M. Bodles-Brakhop, R. Heller, R. Draghia-Akli, Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol. Ther. 17, 585–592 (2009)
D. Bumcrot, M. Manoharan, V. Koteliansky, D. Sah, RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2, 711–719 (2006)
U. Cegovnik, S. Novakovic, Setting optimal parameters for in vitro electrotransfection of B16F1, SA1, LPB, SCK, L929 and CHO cells using predefined exponentially decaying electric pulses. Bioelectrochemistry 62, 73–82 (2004)
K. Cepurniene, P. Ruzgys, R. Treinys, I. Satkauskiene, S. Satkauskas, Influence of plasmid concentration on DNA electrotransfer in vitro using high-voltage and low-voltage pulses. J. Membr. Biol. 236, 81–85 (2010)
R.V. Davalos, D.M. Otten, L.M. Mir, B. Rubinsky, Electrical impedance tomography for imaging tissue electroporation. IEEE Trans. Biomed. Eng. 51, 761–767 (2004)
A. de Fougerolles, H.P. Vornlocher, J. Maraganore, J. Lieberman, Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 6, 443–453 (2007)
E. De Vuyst, M. De Bock, E. Decrock, M. Van Moorhem, C. Naus, C. Mabilde et al., In situ bipolar electroporation for localized cell loading with reporter dyes and investigating gap junctional coupling. Biophys. J. 94, 469–479 (2008)
J.M. Escoffre, T. Portet, L. Wasungu, J. Teissie, D. Dean, M.P. Rols, What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol. Biotechnol. 41, 286–295 (2009)
C. Faurie, M. Rebersek, M. Golzio, M. Kanduser, J.M. Escoffre, M. Pavlin et al., Electro-mediated gene transfer and expression are controlled by the life-time of DNA/membrane complex formation. J. Gen. Med. 12, 117–125 (2010)
Y. Fedorov, A. King, E. Anderson, J. Karpilow, D. Ilsley, W. Marshall et al., Different delivery methods-different expression profiles. Nat. Methods 2, 241–41 (2005)
K.R. Foster, H.P. Schwan, Dielectric properties of tissues—a review, in Handbook of biological effects of electro-magnetic radiation, ed. by C.P.E. Postow, 2nd edn. (CRC Press, Boca Raton, 1995), pp. 25–102
C. García-Martínez, M. Marotta, R. Moore-Carrasco, M. Guitart, M. Camps, S. Busquets et al., Impact on fatty acid metabolism and differential localization of FATP1 and FAT/CD36 proteins delivered in cultured human muscle cells. Am. J. Physiol. Cell Physiol. 288, C1264–C1272 (2005)
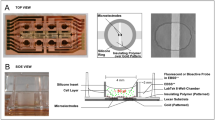
T. Garcia-Sanchez, M. Guitart, J. Rosell, A. MaGomez-Foix, R. Bragos, in Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. Automatic System for Electroporation of Adherent Cells Growing in Standard Multi-Well Plates, vol. pp. 2571–2574, (2012a).
T. Garcia-Sanchez, B. Sanchez-Ortiz, I. Vila, M. Guitart, J. Rosell, A. Gomez-Foix et al., Design and implementation of a microelectrode assembly for use on noncontact in situ electroporation of adherent cells. J. Membr. Biol. 245, 617–624 (2012b)
M. Golzio, J. Teissié, M.P. Rols, Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. 99, 1292–1297 (2002)
T.R. Gowrishankar, J.C. Weaver, An approach to electrical modeling of single and multiple cells. Proc. Natl. Acad. Sci. 100, 3203–3208 (2003)
S. Haberl, M. Kandušer, K. Flisar, D. Hodžić, V.B. Bregar, D. Miklavčič et al., Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gen. Med. 15, 169–181 (2013)
H. He, D.C. Chang, Y.K. Lee, Nonlinear current response of micro electroporation and resealing dynamics for human cancer cells. Bioelectrochemistry 72, 161–168 (2008)
H.L. Huang, H.W. Hsing, T.C. Lai, Y.W. Chen, T.R. Lee, H.T. Chan et al., Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 17, 36 (2010)
H. Huang, Z. Wei, Y. Huang, D. Zhao, L. Zheng, T. Cai et al., An efficient and high-throughput electroporation microchip applicable for siRNA delivery. Lab Chip 11, 163–172 (2011)
A. Ivorra, Tissue Electroporation as a Bioelectric Phenomenon: Basic Concepts, in Irreversible Electroporation, ed. by Rubinsky B, (Springer Berlin Heidelberg, 2010), pp. 23–61
A. Ivorra, B. Al-Sakere, B. Rubinsky, L.M. Mir, In vivo electrical conductivity measurements during and after tumor electroporation: conductivity changes reflect the treatment outcome. Phys. Med. Biol. 54, 5949–5963 (2009)
J. Wegener, C.R. Keese, I. Giaever, Recovery of adherent cells after in situ electroporation monitored electrically. Biotechniques 33, 348, 50, 52 passim (2002)
M. Kanduser, D. Miklavcic, M. Pavlin, Mechanisms involved in gene electrotransfer using high- and low-voltage pulses—an in vitro study. Bioelectrochemistry 74, 265–271 (2009)
C. Kanthou, S. Kranjc, G. Sersa, G. Tozer, A. Zupanic, M. Cemazar, The endothelial cytoskeleton as a target of electroporation-based therapies. Mol. Cancer Ther. 5, 3145–3152 (2006)
K. Kinosita, T.Y. Tsong, Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature 268, 438–441 (1977)
T. Kotnik, G. Pucihar, D. Miklavčič, Induced transmembrane voltage and its correlation with electroporation-mediated molecular transport. J. Membr. Biol. 236, 3–13 (2010)
S. Kwee, H.V. Nielsen, J.E. Celis, Electropermeabilization of human cultured cells grown in monolayers: incorporation of monoclonal antibodies. J. Electroanal. Chem. 298, 65–80 (1990)
A. Liew, F.M. Andre, L.L. Lesueur, M.A. De Menorval, T. O’Brien, L.M. Mir, Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Hum. Gene. Ther. Methods 24, 289–297 (2013)
Y.C. Lin, M. Li, C.S. Fan, L.W. Wu, A microchip for electroporation of primary endothelial cells. Sensors Actuators A Phys. 108, 12–19 (2003)
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001)
I. Marjanovič, S. Haberl, D. Miklavčič, M. Kandušer, M. Pavlin, Analysis and comparison of electrical pulse parameters for gene electrotransfer of two different cell lines. J. Membr. Biol. 236, 97–105 (2010)
L.W. Matthiessen, R.L. Chalmers, D.C.G. Sainsbury, S. Veeramani, G. Kessell, A.C. Humphreys et al., Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 50, 621–629 (2011)
D. Miklavčič, Network for development of electroporation-based technologies and treatments: COST TD1104. J. Membr. Biol. 245, 591–598 (2012)
D. Miklavčič, G. Serša, E. Brecelj, J. Gehl, D. Soden, G. Bianchi et al., Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med. Biol. Eng. Comput. 50, 1213–1225 (2012)
L.M. Mir, Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry 53, 1–10 (2001)
E. Neumann, M. Schaefer-Ridder, Y. Wang, P.H. Hofschneider, Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1, 841–845 (1982)
D.J. Orlicky, J. Schaack, Adenovirus transduction of 3T3-L1 cells. J. Lipid Res. 42, 460–466 (2001)
E. Pasqualotto, A. Ferrario, M. Scaramuzza, A. De Toni, M. Maschietto, Monitoring electropermeabilization of adherent mammalian cells through electrochemical impedance spectroscopy. Procedia Chem. 6, 79–88 (2012)
M. Pavlin, D. Miklavcic, Theoretical and experimental analysis of conductivity, ion diffusion and molecular transport during cell electroporation—relation between short-lived and long-lived pores. Bioelectrochemistry 74, 38–46 (2008)
M. Pavlin, M. Kanduser, M. Rebersek, G. Pucihar, F.X. Hart, R. Magjarevic et al., Effect of cell electroporation on the conductivity of a cell suspension. Biophys. J. 88, 4378–4390 (2005)
M. Rebersek, C. Faurie, M. Kanduser, S. Corovic, J. Teissie, M.P. Rols et al., Electroporator with automatic change of electric field direction improves gene electrotransfer in-vitro. Biomed. Eng. Online 6, 25 (2007)
M.P. Rols, J. Teissié, Experimental evidence for the involvement of the cytoskeleton in mammalian cell electropermeabilization. Biochim. Biophys. Acta Biomembr. 1111, 45–50 (1992)
M.P. Rols, C. Delteil, M. Golzio, J. Teissié, Control by ATP and ADP of voltage-induced mammalian-cell-membrane permeabilization, gene transfer and resulting expression. Eur. J. Biochem. 254, 382–388 (1998)
B. Sanchez, G. Vandersteen, R. Bragos, J. Schoukens, Optimal multisine excitation design for broadband electrical impedance spectroscopy. Meas. Sci. Technol. 22, 115601 (2011)
E. Sarró, M. Lecina, A. Fontova, C. Solà, F. Gòdia, J.J. Cairó et al., Electrical impedance spectroscopy measurements using a four-electrode configuration improve on-line monitoring of cell concentration in adherent animal cell cultures. Biosens. Bioelectron. 31, 257–263 (2012)
T. Shimokawa, K. Okumura, C. Ra, DNA induces apoptosis in electroporated human promonocytic cell line U937. Biochem. Biophys. Res. Commun. 270, 94–99 (2000)
S.I. Sukharev, V.A. Klenchin, S.M. Serov, L.V. Chernomordik, Y.A. Chizmadzhev, Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys. J. 63, 1320–27 (1992)
J. Teissie, M. Golzio, M.P. Rols, Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of ?) knowledge. Biochim. Biophys. Acta Gen. Subj. 1724, 270–280 (2005)
V.F.I. Van Tendeloo, P. Ponsaerts, F. Lardon, G. Nijs, M. Lenjou, C. Van Broeckhoven et al., Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98, 49–56 (2001)
R. Walzem, M. Hickman, J. German, R. Hansen, Transfection of avian LMH-2A hepatoma cells with cationic lipids. Poult. Sci. 76, 882–886 (1997)
T.D. Xie, T.Y. Tsong, Study of mechanisms of electric field-induced DNA transfection. V. Effects of DNA topology on surface binding, cell uptake, expression, and integration into host chromosomes of DNA in the mammalian cell. Biophys. J. 65, 1684–1689 (1993)
Acknowledgements
This study is supported by grants SAF2009-07559 and SAF2012-37480 from the Spanish Ministerio de Ciencia e Innovación (MCI) and CIBERDEM de Diabetes y Enfermedades Metabólicas Asociadas (CB07/08/0012). We also thank Anna Orozco and Alfonso Mendez for their technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
(MP4 9573 kb).
Rights and permissions
About this article
Cite this article
García-Sánchez, T., Guitart, M., Rosell-Ferrer, J. et al. A new spiral microelectrode assembly for electroporation and impedance measurements of adherent cell monolayers. Biomed Microdevices 16, 575–590 (2014). https://doi.org/10.1007/s10544-014-9860-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-014-9860-6