Abstract
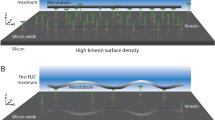
Biomolecular motor proteins have the potential to be used as ‘nano-engines’ for controlled bioseparations and powering nano- and microelectromechanical systems. In order to engineer such systems, biocompatible nanofabrication processes are needed. In this work, we demonstrate an electron beam nanolithography process for patterning kinesin motor proteins. This process was then used to fabricate discontinuous kinesin tracks to study the directionality of microtubule movement under the exclusive influence of surface bound patterned kinesin. Microtubules moved much farther than predicted from a model assuming infinite microtubule stiffness on tracks with discontinuities of 3 μm or less, consistent with a free-end searching mechanism. As the track discontinuities exceeded 3 μm, the measured and predicted propagation distances converged. Observations of partially fixed microtubules suggest that this behavior results from the interaction of the microtubules with the surface and is not governed predominately by the microtubule flexural rigidity.









Similar content being viewed by others
References
L.A. Amos, R.A. Cross, Curr. Opin. Struct. Biol. 7, 239 (1997) doi:10.1016/S0959-440X(97)80032-2
A.K. Boal, H. Tellez, S.B. Rivera, N.E. Miller, G.D. Bachand, B.C. Bunker, Small 2, 793 (2006) doi:10.1002/smll.200500381
T.B. Brown, W.O. Hancock, Nano Lett. 2, 1131 (2002) doi:10.1021/nl025636y
L. Cassimeris, D. Gard, P.T. Tran, H.P. Erickson, J. Cell Sci. 114, 3025 (2001)
L.-J. Cheng, M.-T. Kao, E. Meyhöfer, L.J. Guo, Small 1, 409 (2005) doi:10.1002/smll.200400109
R.K.M. Christie, M.T. Donald, J. Vac. Sci. Technol. A 21, S207 (2003) doi:10.1116/1.1600446
D.L. Coy, M. Wagenbach, J. Howard, J. Biol. Chem. 274, 3667 (1999) doi:10.1074/jbc.274.6.3667
H.G. Craighead, Science 290, 1532 (2000) doi:10.1126/science.290.5496.1532
F. Gittes, B. Mickey, J. Nettleton, J. Howard, J. Cell Biol. 120, 923 (1993) doi:10.1083/jcb.120.4.923
W.O. Hancock, J. Howard, J. Cell Biol. 140, 1395 (1998) doi:10.1083/jcb.140.6.1395
H. Hess, J. Clemmens, C.M. Matzke, G.D. Bachand, B.C. Bunker, V. Vogel, Appl. Phys., A Mater. Sci. Process. 75, 309 (2002) doi:10.1007/s003390201339
Y. Hiratsuka, T. Tada, K. Oiwa, T. Kanayama, T.Q. Uyeda, Biophys. J. 81, 1555 (2001)
J. Howard, Annu. Rev. Physiol 58, 703 (1996) doi:10.1146/annurev.ph.58.030196.003415
A.J. Hunt, J. Howard, Proc. Natl. Acad. Sci. USA 90, 11653 (1993) doi:10.1073/pnas.90.24.11653
A.A. Hyman, J. Cell Sci. Suppl. 14, 125 (1991)
B. Ilic, H.G. Craighead, Biomed. Microdevices 2, 317 (2000) doi:10.1023/A:1009911407093
J. Kerssemakers, J. Howard, H. Hess, S. Diez, Proc. Natl. Acad. Sci. USA 103, 15812 (2006) doi:10.1073/pnas.0510400103
M. Kikumoto, M. Kurachi, V. Tosa, H. Tashiro, Biophys. J. 90, 1687 (2006) doi:10.1529/biophysj.104.055483
F.J. Kull, E.P. Sablin, R. Lau, R.J. Fletterick, R.D. Vale, Nature 380, 550 (1996) doi:10.1038/380550a0
L. Limberis, J.J. Magda, R.J. Stewart, Nano Lett. 1, 277 (2001) doi:10.1021/nl0155375
S.G. Moorjani, L. Jia, T.N. Jackson, W.O. Hancock, Nano Lett. 3, 633 (2003) doi:10.1021/nl034001b
C. Reuther, L. Hajdo, R. Tucker, A.A. Kasprzak, S. Diez, Nano Lett. 6, 2177 (2006) doi:10.1021/nl060922l
W.M. Saxton, M.E. Porter, S.A. Cohn, J.M. Scholey, E.C. Raff, J.R. Mcintosh, Proc. Natl. Acad. Sci. USA 85, 1109 (1988) doi:10.1073/pnas.85.4.1109
K. Svoboda, C.F. Schmidt, B.J. Schnapp, S.M. Block, Nature 365, 721 (1993) doi:10.1038/365721a0
R.D. Vale, R.J. Fletterick, Annu. Rev. Cell Dev. Biol 13, 745 (1997) doi:10.1146/annurev.cellbio.13.1.745
V. VanBuren, L. Cassimeris, D.J. Odde, Biophys. J. 89, 2911 (2005) doi:10.1529/biophysj.105.060913
V. Verma, W. O. Hancock, J. M. Catchmark, Advanced Packaging, IEEE Transactions on [see also Components, Packaging and Manufacturing Technology, Part B: Advanced Packaging, IEEE Transactions on], 28, 584 (2005)
C. Vieu, F. Carcenac, A. Pepin, Y. Chen, M. Mejias, A. Lebib et al., Appl. Surf. Sci. 164, 111 (2000) doi:10.1016/S0169-4332(00)00352-4
F.D. Warner, J.R. McIntosh, Cell movement, Volume 2, kinesin, dynein and microtubule dynamics, 431–440. Pages Liss, New York (1999)
R.C. Williams Jr., J.C. Lee, Methods Enzymol Pt B 85, 376 (1982)
A.P. Yong Chen, Electrophoresis 22, 187 (2001) doi:10.1002/1522-2683(200101)22:2<187::AID-ELPS187>3.0.CO;2-0
Acknowledgements
This work was supported by The Pennsylvania State University Center for Nanoscale Science, a NSF Materials Research Science and Engineering Center (DMR0213623). It was also supported by the National Nanotechnology Infrastructure Network (NSF Cooperative Agreement No. 0335765 with Cornell University) and The Pennsylvania State University Materials Research Institute. V.V wishes to thank the Haythornthwaite Foundation for their Founder’s Prize and grant for year 2005–06.
We thank Maruti Uppalapati for purifying kinesin and Gayatri Muthukrishnan for purifying and labeling tubulin. We also thank Dr. Edward Basgall and Dr. Khalid Eid for assistance with electron beam lithography.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, V., Hancock, W.O. & Catchmark, J.M. Nanoscale patterning of kinesin motor proteins and its role in guiding microtubule motility. Biomed Microdevices 11, 313–322 (2009). https://doi.org/10.1007/s10544-008-9237-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-008-9237-9