Abstract
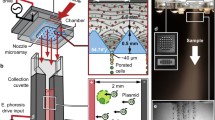
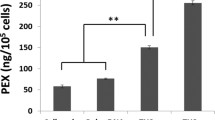
This paper presents a proof-of-concept miniature device for delivery of antisense oligonucleotides (ASO). A piezoelectric, lead zirconate titanate (PZT) plate (0.5 cm2 × 0.75 mm) is used to transfect cells using cavitation-induced sonoporation. Both human umbilical vein endothelial cells (HUVEC) and human prostate cancer cells (PC3) are investigated in vitro. Preliminary results show that after sonication, the transfection rate for HUVEC increases by 96% compared to controls (p < 0.01). For PC3, the transfection rate increases by 31% compared to controls (p < 0.02). This research can potentially be applied in realizing a microelectromechanical system (MEMS)-based device for gene therapy in cancer treatment.
Similar content being viewed by others
References
F. Anderson, Gene therapy: Past, Present and future. Cancer Gene Therapy 10(Supp 1), S13 (2003).
S. Bao, B. Thrall, and D. Miller, Transfection of a reporter plasmid into cultured cells by sonoporaiton in vitro. Ultrasound in Medicine & Biology 23, 953–959 (1997).
J.-R. Bertrand, M. Pottier, A. Vekris, P. Opolon, A. Maksimemko, and C. Malvy, Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochemical and Biophysical Research Communications 296, 1000–1004 (2002).
D. Bufalo, D. Trisciuoglio, M. Scarsella, U. Zangemeister-Wittke, and G. Zupi, Treatment of melanoma cells with a bcl-2/bcl-xl antisense oligonucleotide induces antiangiogenic activity. Oncogene 22, 8441–8447 (2003).
N. Duzgunes, C. de Ilarduya, S. Simoes, R. Zhdanov, K. Konopka, and M. de Lima, Cationic liposomes for gene delivery: Novel cationic lipids and enhancement by proteins and peptides. Current Medicinal Chemistry 10, 1213–1220 (2003).
A. El-Aneed, An overview of current delivery systems in cancer gene therapy. Journal of Controlled Release 94, 1–14 (2004).
M. Fechheimer, J. Boylan, S. Parker, J. Sisken, G. Patel, and S. Zimmer, Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proceeding of National Academy of Science USA 84, 8463–8467 (1987).
L.A. Ferrara, A.J. Fleischman, D. Togawa, T.W. Bauer, E.C. Benzel, and S. Roy, An in vivo biocompatibility assessment of MEMS materials for spinal fusion monitoringSource. Biomedical Microdevices 5, 297–302 (2003).
S. Gambihler, M. Delius, and E.J.W., Permeabilization of the plasma membrane of l1210 mouse leukemia cells using lithotripter shock waves. The Journal of Membrane Biology 141, 267–275 (1994).
M. Gleave and H. Miyake, Use of antisense oligonucleotides targeting the cytoprotective gene, clusterin, to enhance androgen- and chemo-sensitivity in prostate cancer. World Journal of Urology (2005).
P. Huber, et al., In vitro and in vivo transfection of plasmid DNA in the dunning prostate tumor r3327-at1 is enhanced by focused ultrasound. Gene Therapy 7(17), 1516–1525 (2000).
K. Ikuta, Y. Sasaki, H. Maegawa, and S. Maruo, Biochemical ic chip for pretreatment in biochemical experiments. In Proceedings of IEEE Micro Electro Mechanical Systems (2003).
G. Kotzara, M. Freas, P, Abelb, A. Fleischmanc, S. Royc, C. Zormand, J.M. Morane, and J. Melzakd, Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 23, 2737–2750 (2002).
A. Miller, C. Dou, and J. Song, DNA transfer and cell killing in epidermoid cells by diagnostic ultrasound activation of contrast agent gas bodies in vitro. Ultrasound in Medicine & Biology 29, 601–607 (2003).
D. Miller, S. Pislaru, and J. Greenleaf, Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somatic Cell amd Molecular Genetics 27, 115–134 (2002).
A. Miller and J. Quddus, Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound in Medicine & Biology 26, 661–667 (2000).
A. Miller and J. Song, Tumor growth reduction and DNA transfer by cavitation-enhanced high-intensity focused ultrasound in vivo. Ultrasound in Medicine & Biology 29, 887–893 (2003).
M. Nesterova and Y. Cho-Chung, Killing the messenger: Antisense DNA and siRNA. Current Drug Targets 5, 683–689 (2004).
T. Niidome and L. Huang, Gene therapy progress and prospects: Nonviral vectors. Gene Therapy 9, 1647–1652 (2002).
C. Ohl and B. Wolfrum, Detachment and sonoporation of adherent hela-cells by shock wave-induced cavitation. Biochimica et Biophysica Acta 1624, 131–138 (2003).
D. Rabussay, B. Dev, F. Jason, S. Georg, and Z. Lei, Enhancement of therapeutic drug and DNA delivery into cells by electroporation. Journal of Physics D: Applied Physics 36, 348–363 (2003).
J. Santini, M. Cima, and R. Langer, A controlled-release microchip. Nature 397, 335–338 (1999).
M. Sohn, R.L. Murray, K. Schwarz, J. Nyitray, P. Purray, A. Franko, K. Palmer, L. Diebel, and S. Dulchavsky, In-vivo particle mediated delivery of mRNA to mammalian tissues: ballistic and biologic effects. Wound Repair and Regeneration 9(4), 287–296 (2001).
J. Song, D. Tata, L. Li, J. Taylor, S. Bao, and D. L. Miller, Combined shock-wave and immunogene therapy of mouse melanoma and renal carcinoma tumors. Ultrasound in Medicine & Biology 28, 957–964 (2002).
J. Sundaram, B. Mellein, and S. Mitragotri, An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophysical Journal 84, 3087–3101 (2003).
J. Tousignant, A. Gates, L. Ingram, C. Johnson, J. Nietupski, S. Cheng, S. Eastman, and R. Scheule, Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Human Gene Therapy 11, 2493–2513 (2000).
K. Tschoep, G. Hartmann, R. Jox, S. Thompson, A. Eigler, A. Krug, S. Erhardt, G. Adams, S. Endres, and M. Delius, Shock waves: a novel method for cytoplasmic delivery of antisense oligonucleotides. Journal of Molecular Medicine 79, 306–313 (2001).
I. Verma and N. Somia, Gene therapy-promises, problems and prospects. Nature 389, 239–242 (1997).
Z. Yang, S. Matsumoto, and R. Maeda, A prototype of ultrasonic micro-degassing device for portable dialysis system. Sensors and Actuators A 95, 274–280 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this paper was presented at the 13th International Conference on Solid-State Sensors and Actuators (Transducers’ 05), June, 2005.
Rights and permissions
About this article
Cite this article
Siu, T., Rohling, R. & Chiao, M. Microdevice-based delivery of gene products using sonoporation. Biomed Microdevices 9, 295–300 (2007). https://doi.org/10.1007/s10544-006-9028-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-006-9028-0