Abstract
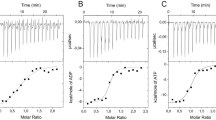
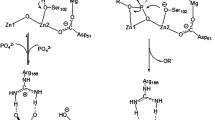
Soluble inorganic pyrophosphatase from Escherichia coli (E-PPase) is a hexamer forming under acidic conditions the active trimers. We have earlier found that the hydrolysis of a substrate (MgPPi) by the trimers as well as a mutant E-PPase Asp26Ala did not obey the Michaelis-Menten equation. To explain this fact, a model has been proposed implying the existence of, aside from an active site, an effector site that can bind PPi and thus accelerate MgPPi hydrolysis. In this paper, we demonstrate that the noncompetitive activation of MgPPi hydrolysis by metal-free PPi can also explain kinetic features of hexameric forms of both the native enzyme and the specially obtained mutant E-PPase with a substituted residue Glu145 in a flexible loop 144–149. Aside from PPi, its non-hydrolyzable analog methylene diphosphonate can also occupy the effector site resulting in the acceleration of the substrate hydrolysis. Our finding that two moles of [32P]PPi can bind with each enzyme subunit is direct evidence for the existence of the effector site in the native E-PPase.
Similar content being viewed by others
REFERENCES
Eliasson, R., Ponbis, E., Sun, X., and Richards, P. (1994) J. Biol. Chem., 269, 26052–26057.
Kobe, B., Jennings, F. G., House, C. M., Michell, B. J., Goodwill, K. E., Santarsiero, B. D., Stevens, K. C., Cotton, R. G. H., and Kemp, B. E. (1999) Nat. Struct. Biol., 6, 442–448.
Masson, P., Schopfer, S. M., Barbels, C. F., Fromenb, F.-T., Ribes, F., Nachon, F., and Lockridge, O. (2002) Biochim. Biophys. Acta, 1594, 313–324.
Baburina, I., Dikdan, G., Guo, F., Tous, G. I., Roof, B., and Jordan, F. (1998) Biochemistry, 37, 1245–1255.
Karsten, W., Pais, J., Rao, G., Harris, B., and Cook, P. (2002) Biochemistry, 42, 9712–9721.
Oganessyan, V. Yu., Kurilova, S. A., Vorobyeva, N. N., Nazarova, T. I., Popov, A. N., Lebedev, A. A., Avaeva, S. M., and Harutyunyan, E. H. (1994) FEBS Lett., 348, 301–304.
Harutyunyan, E. H., Oganessyan, V. Yu., Oganessyan, N. N., Avaeva, S. M., Nazarova, T. I., Vorobyeva, N. N., Kurilova, S. A., Huber, R., and Mather, T. (1997) Biochemistry, 36, 7754–7760.
Kankare, J., Salminen, T., Lahti, R., Cooperman, B. S., Baykov, A. A., and Goldman, A. (1996) Biochemistry, 35, 4670–4677.
Arutyunyan, E. G., Oganessyan, V. Yu., Oganessyan, N. N., Terzyan, S. S., Popov, A. N., Rubinsky, S. V., Vainshtein, B. K., Nazarova, T. I., Kurilova, S. A., Vorobyeva, N. N., and Avaeva, S. M. (1996) Kristallografiya, 41, 84–96.
Samygina, V. R., Popov, A. N., Rodina, E. V., Vorobyeva, N. N., Lamzin, V. S., Polyakov, K. M., Kurilova, S. A., Nazarova, T. I., and Avaeva, S. M. (2001) J. Mol. Biol., 314, 633–645.
Avaeva, S., Kurilova, S., Nazarova, T., Rodina, E., Vorobyeva, N., Sklyankina, V., Grigorjeva, O., Harutyunyan, E., Ogannesyan, V., Wilson, K., Dauter, Z., Huber, R., and Mather, T. (1997) FEBS Lett., 410, 502–508.
Avaeva, S. M., Rodina, E. V., Kurilova, S. A., Nazarova, T. I., and Vorobyeva, N. N. (1996) FEBS Lett., 392, 91–94.
Leppanen, V.-M., Nummelin, H., Hansen, T., Lahti, R., Schafer, J., and Goldman, A. (1999) Protein Sci., 8, 1218–1231.
Avaeva, S., Grigorjeva, O., Mitkevich, V., Sklyankina, V., and Varfolomeyev, S. (1999) FEBS Lett., 464, 169–173.
Sitnik, T. S., Vainonen, J. P., Rodina, E. V., Nazarova, T. I., Kurilova, S. A., Vorobyeva, N. N., and Avaeva, S. M. (2003) IUBMB Life, 55, 37–41.
Kraulis, P. (1991) J. Appl. Crystallogr., 24, 946–950.
Avaeva, S., Ignatov, P., Kurilova, S., Nazarova, T., Rodina, E., Vorobyeva, N., Ogannesyan, V., and Harutyunyan, E. (1996) FEBS Lett., 399, 99–102.
Josse, J. (1966) J. Biol. Chem., 241, 1938–1947.
Baykov, A. A., and Avaeva, S. M. (1981) Analyt. Biochem., 116, 1–4.
Vainonen, Yu. P., Kurilova, S. A., and Avaeva, S. M. (2002) Bioorg. Khim., 28, 426–433.
Sutherland, G. R. J., and Aust, S. D. (1997) Biochemistry, 36, 8567–8573.
Avaeva, S. M., Rodina, E. V., Kurilova, S. A., Nazarova, T. I., Vorobyeva, N. N., Harutyunyan, E. H., and Oganessyan, V. Y. (1995) FEBS Lett., 377, 44–46.
Chervenka, C. H. (1972) Methods for Analytical Ultracentrifuge, Spinco Division of Beckman Instruments, Inc., Palo Alto.
Pohjanjoki, P., Lahti, R., Goldman, A., and Cooperman, B. S. (1998) Biochemistry, 37, 1754–1761.
Smirnova, I. N., Kudryavtseva, N. A., Komissarenko, S. V., Tarusova, N. B., and Baykov, A. A. (1988) Arch. Biochem. Biophys., 267, 280–284.
Avaeva, S. M., Vorobjeva, N. N., Kurilova, S. A., Nazarova, T. I., Polyakov, K. M., Rodina, E. V., and Samygina, V. R. (2000) Biochemistry (Moscow), 65, 373–387.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Biokhimiya, Vol. 70, No. 1, 2005, pp. 85–96.
Original Russian Text Copyright © 2005 by Vainonen, Vorobyeva, Rodina, Nazarova, Kurilova, Skoblov, Avaeva.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM04-072, June 27, 2004.
Rights and permissions
About this article
Cite this article
Vainonen, J.P., Vorobyeva, N.N., Rodina, E.V. et al. Metal-free PPi activates hydrolysis of MgPPi by an Escherichia coli inorganic pyrophosphatase. Biochemistry (Moscow) 70, 69–78 (2005). https://doi.org/10.1007/s10541-005-0053-z
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0053-z